Cisplatin chemotherapy and renal function
- PMID: 34353441
- PMCID: PMC8963537
- DOI: 10.1016/bs.acr.2021.03.008
Cisplatin chemotherapy and renal function
Abstract
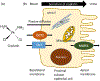
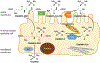
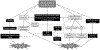
Cisplatin has been a mainstay of cancer chemotherapy since the 1970s. Despite its broad anticancer potential, its clinical use has regularly been constrained by kidney toxicities. This review details those biochemical pathways and metabolic conversions that underlie the kidney toxicities. A wide range of redox events contribute to the eventual physiological consequences of drug activities.
Keywords: Alkylating agent; Antioxidants; Bioactivation; Cisplatin; Glutathione; Glutathione S-transferases; Kidney; Nephrotoxicity; Redox pathways; Transporters.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- Arjumand W, & Sultana S (2011). Glycyrrhizic acid: A phytochemical with a protective role against cisplatin-induced genotoxicity and nephrotoxicity. Life Sciences, 89, 422–429. - PubMed
-
- Bakir S, Yazgan UC, Ibiloglu I, Elbey B, Kizil M, & Kelle M (2015). The protective effect of pomegranate extract against cisplatin toxicity in rat liver and kidney tissue. Archives of Physiology and Biochemistry, 121, 152–156. - PubMed
-
- Boroushaki MT, Rajabian A, Farzadnia M, Hoseini A, Poorlashkari M, Taghavi A, et al. (2015). Protective effect of pomegranate seed oil against cisplatin-induced nephrotoxicity in rat. Renal Failure, 37, 1338–1343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

