Pharmacological Characterization of the Imipridone Anticancer Drug ONC201 Reveals a Negative Allosteric Mechanism of Action at the D2 Dopamine Receptor
- PMID: 34353882
- PMCID: PMC8626643
- DOI: 10.1124/molpharm.121.000336
Pharmacological Characterization of the Imipridone Anticancer Drug ONC201 Reveals a Negative Allosteric Mechanism of Action at the D2 Dopamine Receptor
Abstract
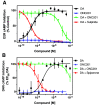
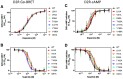
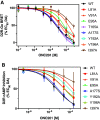
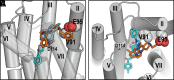
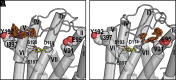
ONC201 is a first-in-class imipridone compound that is in clinical trials for the treatment of high-grade gliomas and other advanced cancers. Recent studies identified that ONC201 antagonizes D2-like dopamine receptors at therapeutically relevant concentrations. In the current study, characterization of ONC201 using radioligand binding and multiple functional assays revealed that it was a full antagonist of the D2 and D3 receptors (D2R and D3R) with low micromolar potencies, similar to its potency for antiproliferative effects. Curve-shift experiments using D2R-mediated β-arrestin recruitment and cAMP assays revealed that ONC201 exhibited a mixed form of antagonism. An operational model of allostery was used to analyze these data, which suggested that the predominant modulatory effect of ONC201 was on dopamine efficacy with little to no effect on dopamine affinity. To investigate how ONC201 binds to the D2R, we employed scanning mutagenesis coupled with a D2R-mediated calcium efflux assay. Eight residues were identified as being important for ONC201's functional antagonism of the D2R. Mutation of these residues followed by assessing ONC201 antagonism in multiple signaling assays highlighted specific residues involved in ONC201 binding. Together with computational modeling and simulation studies, our results suggest that ONC201 interacts with the D2R in a bitopic manner where the imipridone core of the molecule protrudes into the orthosteric binding site, but does not compete with dopamine, whereas a secondary phenyl ring engages an allosteric binding pocket that may be associated with negative modulation of receptor activity. SIGNIFICANCE STATEMENT: ONC201 is a novel antagonist of the D2 dopamine receptor with demonstrated efficacy in the treatment of various cancers, especially high-grade glioma. This study demonstrates that ONC201 antagonizes the D2 receptor with novel bitopic and negative allosteric mechanisms of action, which may explain its high selectivity and some of its clinical anticancer properties that are distinct from other D2 receptor antagonists widely used for the treatment of schizophrenia and other neuropsychiatric disorders.
U.S. Government work not protected by U.S. copyright.
Figures
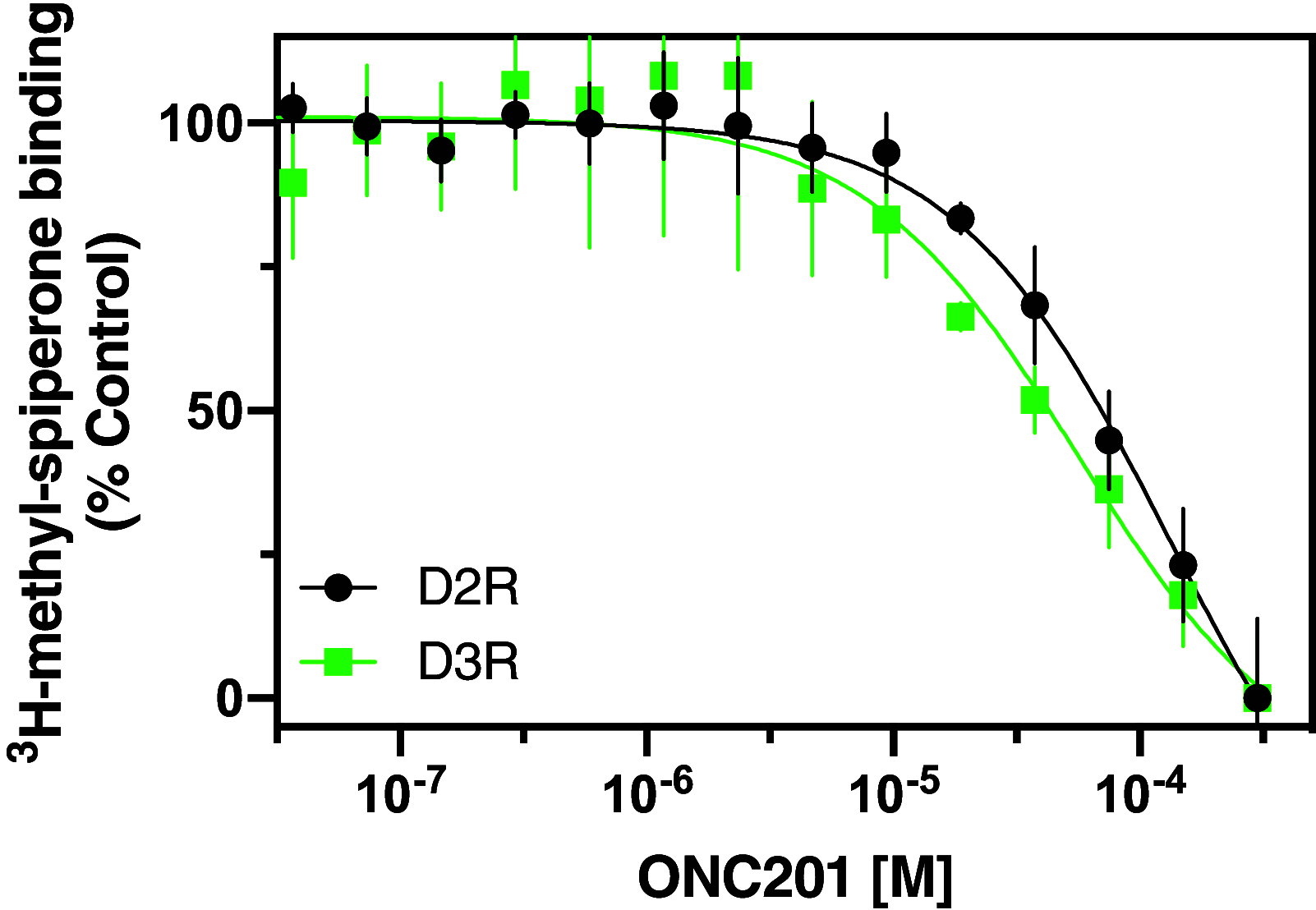



References
-
- Ballesteros JA, Weinstein H (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods in Neuroscience 25:366–428.
-
- Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

