Maturation of the matrix and viral membrane of HIV-1
- PMID: 34353956
- PMCID: PMC7611776
- DOI: 10.1126/science.abe6821
Maturation of the matrix and viral membrane of HIV-1
Abstract
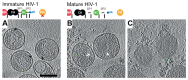
Gag, the primary structural protein of HIV-1, is recruited to the plasma membrane for virus assembly by its matrix (MA) domain. Gag is subsequently cleaved into its component domains, causing structural maturation to repurpose the virion for cell entry. We determined the structure and arrangement of MA within immature and mature HIV-1 through cryo-electron tomography. We found that MA rearranges between two different hexameric lattices upon maturation. In mature HIV-1, a lipid extends out of the membrane to bind with a pocket in MA. Our data suggest that proteolytic maturation of HIV-1 not only assembles the viral capsid surrounding the genome but also repurposes the membrane-bound MA lattice for an entry or postentry function and results in the partial removal of up to 2500 lipids from the viral membrane.
Copyright © 2021, American Association for the Advancement of Science.
Conflict of interest statement
Authors declare no competing interests.
Figures



Comment in
-
Maturation of HIV-1.Science. 2021 Aug 6;373(6555):621-622. doi: 10.1126/science.abj9075. Science. 2021. PMID: 34353938 No abstract available.
References
-
- Mattei S, Schur FK, Briggs JAG. Retrovirus maturation — an extraordinary structural transformation. Current Opinion in Virology. 2016;18:27–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

