Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic
- PMID: 34354082
- PMCID: PMC8342436
- DOI: 10.1038/s41541-021-00356-x
Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic
Abstract
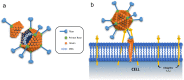
Adenoviral vectors have been explored as vaccine agents for a range of infectious diseases, and their ability to induce a potent and balanced immune response made them logical candidates to apply to the COVID-19 pandemic. The unique molecular characteristics of these vectors enabled the rapid development of vaccines with advanced designs capable of overcoming the biological challenges faced by early adenoviral vector systems. These successes and the urgency of the COVID-19 situation have resulted in a flurry of candidate adenoviral vector vaccines for COVID-19 from both academia and industry. These vaccines represent some of the lead candidates currently supported by Operation Warp Speed and other government agencies for rapid translational development. This review details adenoviral vector COVID-19 vaccines currently in human clinical trials and provides an overview of the new technologies employed in their design. As these vaccines have formed a cornerstone of the COVID-19 global vaccination campaign, this review provides a full consideration of the impact and development of this emerging platform.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

