Restricted differentiative capacity of Wt1-expressing peritoneal mesothelium in postnatal and adult mice
- PMID: 34354169
- PMCID: PMC8342433
- DOI: 10.1038/s41598-021-95380-1
Restricted differentiative capacity of Wt1-expressing peritoneal mesothelium in postnatal and adult mice
Abstract
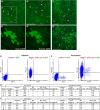
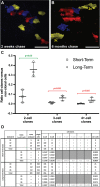
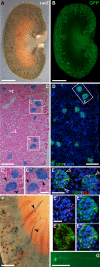
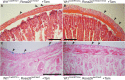
Previously, genetic lineage tracing based on the mesothelial marker Wt1, appeared to show that peritoneal mesothelial cells have a range of differentiative capacities and are the direct progenitors of vascular smooth muscle in the intestine. However, it was not clear whether this was a temporally limited process or continued throughout postnatal life. Here, using a conditional Wt1-based genetic lineage tracing approach, we demonstrate that the postnatal and adult peritoneum covering intestine, mesentery and body wall only maintained itself and failed to contribute to other visceral tissues. Pulse-chase experiments of up to 6 months revealed that Wt1-expressing cells remained confined to the peritoneum and failed to differentiate into cellular components of blood vessels or other tissues underlying the peritoneum. Our data confirmed that the Wt1-lineage system also labelled submesothelial cells. Ablation of Wt1 in adult mice did not result in changes to the intestinal wall architecture. In the heart, we observed that Wt1-expressing cells maintained the epicardium and contributed to coronary vessels in newborn and adult mice. Our results demonstrate that Wt1-expressing cells in the peritoneum have limited differentiation capacities, and that contribution of Wt1-expressing cells to cardiac vasculature is based on organ-specific mechanisms.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

