Neocortical inhibitory interneuron subtypes are differentially attuned to synchrony- and rate-coded information
- PMID: 34354206
- PMCID: PMC8342442
- DOI: 10.1038/s42003-021-02437-y
Neocortical inhibitory interneuron subtypes are differentially attuned to synchrony- and rate-coded information
Abstract
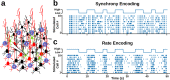
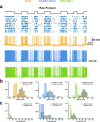
Neurons can carry information with both the synchrony and rate of their spikes. However, it is unknown whether distinct subtypes of neurons are more sensitive to information carried by synchrony versus rate, or vice versa. Here, we address this question using patterned optical stimulation in slices of somatosensory cortex from mouse lines labelling fast-spiking (FS) and regular-spiking (RS) interneurons. We used optical stimulation in layer 2/3 to encode a 1-bit signal using either the synchrony or rate of activity. We then examined the mutual information between this signal and the interneuron responses. We found that for a synchrony encoding, FS interneurons carried more information in the first five milliseconds, while both interneuron subtypes carried more information than excitatory neurons in later responses. For a rate encoding, we found that RS interneurons carried more information after several milliseconds. These data demonstrate that distinct interneuron subtypes in the neocortex have distinct sensitivities to synchrony versus rate codes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

