Fluorescence/photoacoustic imaging-guided nanomaterials for highly efficient cancer theragnostic agent
- PMID: 34354208
- PMCID: PMC8342712
- DOI: 10.1038/s41598-021-95660-w
Fluorescence/photoacoustic imaging-guided nanomaterials for highly efficient cancer theragnostic agent
Erratum in
-
Author Correction: Fluorescence/photoacoustic imaging-guided nanomaterials for highly efficient cancer theragnostic agent.Sci Rep. 2021 Sep 8;11(1):18210. doi: 10.1038/s41598-021-98159-6. Sci Rep. 2021. PMID: 34497353 Free PMC article. No abstract available.
Abstract
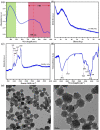
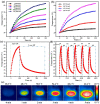
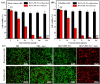
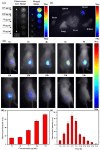
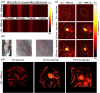
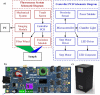
Imaging modalities combined with a multimodal nanocomposite contrast agent hold great potential for significant contributions in the biomedical field. Among modern imaging techniques, photoacoustic (PA) and fluorescence (FL) imaging gained much attention due to their non-invasive feature and the mutually supportive characteristic in terms of spatial resolution, penetration depth, imaging sensitivity, and speed. In this present study, we synthesized IR783 conjugated chitosan-polypyrrole nanocomposites (IR-CS-PPy NCs) as a theragnostic agent used for FL/PA dual-modal imaging. A customized FL and photoacoustic imaging system was constructed to perform required imaging experiments and create high-contrast images. The proposed nanocomposites were confirmed to have great biosafety, essentially a near-infrared (NIR) absorbance property with enhanced photostability. The in vitro photothermal results indicate the high-efficiency MDA-MB-231 breast cancer cell ablation ability of IR-CS-PPy NCs under 808 nm NIR laser irradiation. The in vivo PTT study revealed the complete destruction of the tumor tissues with IR-CS-PPy NCs without further recurrence. The in vitro and in vivo results suggest that the demonstrated nanocomposites, together with the proposed imaging systems could be an effective theragnostic agent for imaging-guided cancer treatment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, Liu B, Lei Y, Du S, Vuppalapati A, Luu HH, Haydon RC, He TC, Ren G. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106. doi: 10.1016/j.gendis.2018.05.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

