Revisiting the neurovascular unit
- PMID: 34354283
- PMCID: PMC9462551
- DOI: 10.1038/s41593-021-00904-7
Revisiting the neurovascular unit
Abstract
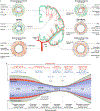
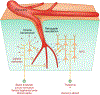
The brain is supplied by an elaborate vascular network that originates extracranially and reaches deep into the brain. The concept of the neurovascular unit provides a useful framework to investigate how neuronal signals regulate nearby microvessels to support the metabolic needs of the brain, but it does not consider the role of larger cerebral arteries and systemic vasoactive signals. Furthermore, the recently emerged molecular heterogeneity of cerebrovascular cells indicates that there is no prototypical neurovascular unit replicated at all levels of the vascular network. Here, we examine the cellular and molecular diversity of the cerebrovascular tree and the relative contribution of systemic and brain-intrinsic factors to neurovascular function. Evidence supports the concept of a 'neurovascular complex' composed of segmentally diverse functional modules that implement coordinated vascular responses to central and peripheral signals to maintain homeostasis of the brain. This concept has major implications for neurovascular regulation in health and disease and for brain imaging.
© 2021. Springer Nature America, Inc.
Figures
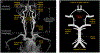

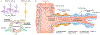

References
-
- Alves de Lima K, Rustenhoven J & Kipnis J Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol 38, 597–620 (2020). - PubMed
-
- Paredes I, Himmels P & Ruiz de Almodovar C Neurovascular communication during CNS development. Dev. Cell 45, 10–32 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

