Remimazolam: Non-Clinical and Clinical Profile of a New Sedative/Anesthetic Agent
- PMID: 34354587
- PMCID: PMC8329483
- DOI: 10.3389/fphar.2021.690875
Remimazolam: Non-Clinical and Clinical Profile of a New Sedative/Anesthetic Agent
Abstract
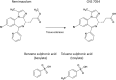
A program to identify novel intravenous sedatives with a short and predictable duration of action was initiated in the late 1990's by Glaxo Wellcome. The program focussed on the identification of ester-based benzodiazepine derivatives that are rapidly broken down by esterases. Remimazolam was identified as one of the lead compounds. The project at Glaxo was shelved for strategic reasons at the late lead optimization stage. Via the GSK ventures initiative, the program was acquired by the small biotechnology company, TheraSci, and, through successive acquisitions, developed as the besylate salt at CeNeS and PAION. The development of remimazolam besylate has been slow by industry standards, primarily because of the resource limitations of these small companies. It has, however, recently been approved for anesthesia in Japan and South Korea, procedural sedation in the United States, China, and Europe, and for compassionate use in intensive care unit sedation in Belgium. A second development program of remimazolam was later initiated in China, using a slightly different salt form, remimazolam tosylate. This salt form of the compound has also recently been approved for procedural sedation in China. Remimazolam has the pharmacological profile of a classical benzodiazepine, such as midazolam, but is differentiated from other intravenous benzodiazepines by its rapid conversion to an inactive metabolite resulting in a short onset/offset profile. It is differentiated from other intravenous hypnotic agents, such as propofol, by its low liability for cardiovascular depression, respiratory depression, and injection pain. The benzodiazepine antagonist flumazenil can reverse the effects of remimazolam in case of adverse events and further shorten recovery times. The aim of this review is to provide an analysis of, and perspective on, published non-clinical and clinical information on 1) the pharmacology, metabolism, pharmacokinetics, and pharmacodynamic profile of remimazolam, 2) the profile of remimazolam compared with established agents, 3) gaps in the current understanding of remimazolam, 4) the compound's discovery and development process and 5) likely future developments in the clinical use of remimazolam.
Keywords: anesthesia; benzodiazepine; midazolam; propofol; remimazolam; sedation; short-acting anesthetic; total intravenous anesthesia.
Copyright © 2021 Kilpatrick.
Conflict of interest statement
The author is a director and employee of GJK Pharma Ltd.
Figures


References
-
- Antonik L. J., Goldwater D. R., Kilpatrick G. J., Tilbrook G. S., Borkett K. M. (2012). A Placebo- and Midazolam-Controlled Phase I Single Ascending-Dose Study Evaluating the Safety, Pharmacokinetics, and Pharmacodynamics of Remimazolam (CNS 7056). Anesth. Analgesia 115 (2), 274–283. 10.1213/ANE.0b013e31823f0c28 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

