Rapalink-1 Increased Infarct Size in Early Cerebral Ischemia-Reperfusion With Increased Blood-Brain Barrier Disruption
- PMID: 34354602
- PMCID: PMC8329705
- DOI: 10.3389/fphys.2021.706528
Rapalink-1 Increased Infarct Size in Early Cerebral Ischemia-Reperfusion With Increased Blood-Brain Barrier Disruption
Abstract
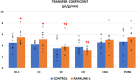
It has been reported that the mechanistic target of rapamycin (mTOR) pathway is involved in cerebral ischemia-reperfusion injury. One of the important pathological changes during reperfusion after cerebral ischemia is disruption of blood-brain barrier (BBB). Rapamycin, a first-generation mTOR inhibitor, produces divergent effects on neuronal survival and alteration in BBB disruption. In this study, we investigated how Rapalink-1, a third-generation mTOR inhibitor, would affect neuronal survival and BBB disruption in the very early stage of cerebral ischemia-reperfusion that is within the time window of thrombolysis therapy. The middle cerebral artery occlusion (MCAO) was performed in rats under isoflurane anesthesia with controlled ventilation. Of note, 2 mg/kg of Rapalink-1 or vehicle was administered intraperitoneally 10 min after MCAO. After 1 h of MCAO and 2 h of reperfusion, the transfer coefficient (Ki) of 14C-α-aminoisobutyric acid (104 Da) and the volume of 3H-dextran (70,000 Da) distribution were determined to assess the degree of BBB disruption. At the same time points, phosphorylated S6 (Ser240/244) and Akt (Ser473) as well as matrix metalloproteinase-2 (MMP2) protein level were determined by Western blot along with the infarct size using tetrazolium stain. Rapalink-1 increased the Ki in the ischemic-reperfused cortex (IR-C, +23%, p < 0.05) without a significant change in the volume of dextran distribution. Rapalink-1 increased the percentage of cortical infarct out of the total cortical area (+41%, p < 0.005). Rapalink-1 significantly decreased phosphorylated S6 and Akt to half the level of the control rats in the IR-C, which suggests that both of the mechanistic target of rapamycin complex 1 and 2 (mTORC1 and mTORC2) were inhibited. The MMP2 level was increased suggesting that BBB disruption could be aggravated by Rapalink-1. Taken together, our data suggest that inhibiting both mTORC1 and mTORC2 by Rapalink-1 could worsen the neuronal damage in the early stage of cerebral ischemia-reperfusion and that the aggravation of BBB disruption could be one of the contributing factors.
Keywords: 14C-α-aminoisobutyric acid; Rapalink-1; blood-brain barrer; brain protection; cerebral ischemia-reperfusion; mTOR inhibitor.
Copyright © 2021 Chi, Liu, Cofano, Patel, Jacinto and Weiss.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Comment in
-
Commentary: Rapalink-1 Increased Infarct Size in Early Cerebral Ischemia-Reperfusion With Increased Blood-Brain Barrier Disruption.Front Physiol. 2021 Sep 22;12:761556. doi: 10.3389/fphys.2021.761556. eCollection 2021. Front Physiol. 2021. PMID: 34630168 Free PMC article. No abstract available.
References
-
- Beard D. J., Li Z., Schneider A. M., Couch Y., Cipolla M. J., Buchan A. M. (2020). Rapamycin induces an eNOS (endothelial nitric oxide synthase) dependent increase in brain collateral perfusion in wistar and spontaneously hypertensive rats. Stroke 51, 2834–2843. 10.1161/STROKEAHA.120.029781 - DOI - PMC - PubMed
-
- Boulanger J. M., Lindsay M. P., Gubitz G., Smith E. E., Stotts G., Foley N., et al. . (2018). Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int. J. Stroke Off. J. Int. Stroke Soc. 13, 949–984. 10.1177/1747493018786616 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

