Insight Into Polysaccharides From Panax ginseng C. A. Meyer in Improving Intestinal Inflammation: Modulating Intestinal Microbiota and Autophagy
- PMID: 34354704
- PMCID: PMC8329555
- DOI: 10.3389/fimmu.2021.683911
Insight Into Polysaccharides From Panax ginseng C. A. Meyer in Improving Intestinal Inflammation: Modulating Intestinal Microbiota and Autophagy
Abstract
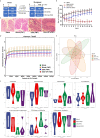
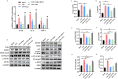
Polysaccharides from Panax ginseng C. A. Meyer (P. ginseng) are the main active component of P. ginseng and exhibit significant intestinal anti-inflammatory activity. However, the therapeutic mechanism of the ginseng polysaccharide is unclear, and this hinders the application for medicine or functional food. In this study, a polysaccharide was isolated from P. ginseng (GP). The primary structure and morphology of the GP were studied by HPLC, FT-IR spectroscopy, and scanning electron microscopy (SEM). Further, its intestinal anti-inflammatory activity and its mechanism of function were evaluated in experimental systems using DSS-induced rats, fecal microbiota transplantation (FMT), and LPS-stimulated HT-29 cells. Results showed that GP modulated the structure of gut microbiota and restored mTOR-dependent autophagic dysfunction. Consequently, active autophagy suppressed inflammation through the inhibition of NF-κB, oxidative stress, and the release of cytokines. Therefore, our research provides a rationale for future investigations into the relationship between microbiota and autophagy and revealed the therapeutic potential of GP for inflammatory bowel disease.
Keywords: Panax ginseng C. A. Meyer; autophagy; gut microbiota; intestinal inflammation; polysaccharide.
Copyright © 2021 Wang, Shao, Zhang, Zhao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Di Lorenzo F, Kubik Ł., Oblak A, Lorè NI, Cigana C, Lanzetta R, et al. . Activation of Human Toll-Like Receptor 4 (TLR4)·Myeloid Differentiation Factor 2 (MD-2) by Hypoacylated Lipopolysaccharide From a Clinical Isolate of Burkholderia Cenocepacia. J Biol Chem (2015) 290(35):21305–19. 10.1074/jbc.M115.649087 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

