Development of a Human Intestinal Organoid Model for In Vitro Studies on Gut Inflammation and Fibrosis
- PMID: 34354753
- PMCID: PMC8331310
- DOI: 10.1155/2021/9929461
Development of a Human Intestinal Organoid Model for In Vitro Studies on Gut Inflammation and Fibrosis
Abstract
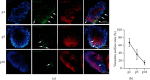
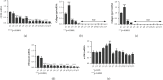
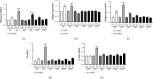
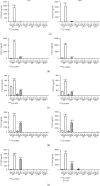
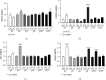
Inflammatory Bowel Diseases (IBDs) are characterized by chronic intestinal inflammation and fibrosis, the latter being the predominant denominator for long-term complications. Epithelial and mesenchymal 2D cultures are highly utilized in vitro models for the preclinical evaluation of anti-inflammatory and antifibrotic therapies. More recently, human intestinal organoids (HIOs), a new 3D in vitro model derived from pluripotent stem cells, have the advantage to closely resemble the architecture of the intestinal mucosa. However, the appropriate timing for the study of inflammatory and fibrotic responses, during HIO development, has not been adequately investigated. We developed HIOs from the human embryonic stem cell line, H1, and examined the expression of mesenchymal markers during their maturation process. We also investigated the effect of inflammatory stimuli on the expression of fibrotic and immunological mediators. Serial evaluation of the expression of mesenchymal and extracellular matrix (ECM) markers revealed that HIOs have an adequately developed mesenchymal component, which gradually declines through culture passages. Specifically, CD90, collagen type I, collagen type III, and fibronectin were highly expressed in early passages but gradually diminished in late passages. The proinflammatory cytokines IL-1α and TNF-α induced the mRNA expression of fibronectin, collagen types I and III, tissue factor (TF), and alpha-smooth muscle actin (α-SMA) primarily in early passages. Similarly, HIOs elicited strong mRNA and protein mesenchymal (CXCL10) and epithelial (CXCL1, CCL2, CXCL8, and CCL20) chemokine responses in early but not late passages. In contrast, the epithelial tight junction components, CLDN1 and JAMA, responded to inflammatory stimulation independently of the culture passage. Our findings indicate that this HIO model contains a functional mesenchymal component, during early passages, and underline the significance of the mesenchymal cells' fitness in inflammatory and fibrotic responses. Therefore, we propose that this model is suitable for the study of epithelial-mesenchymal interactions in early passages when the mesenchymal component is active.
Copyright © 2021 Leonidas Kandilogiannakis et al.
Conflict of interest statement
The authors have no conflict to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

