A novel selective AKR1C3-activated prodrug AST-3424/OBI-3424 exhibits broad anti-tumor activity
- PMID: 34354865
- PMCID: PMC8332853
A novel selective AKR1C3-activated prodrug AST-3424/OBI-3424 exhibits broad anti-tumor activity
Erratum in
-
Erratum: A novel selective AKR1C3-activated prodrug AST-3424/OBI-3424 exhibits broad anti-tumor activity.Am J Cancer Res. 2023 Nov 15;13(11):5750. eCollection 2023. Am J Cancer Res. 2023. PMID: 38058837 Free PMC article.
Abstract
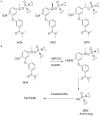
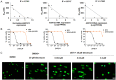
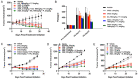
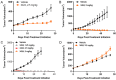
AST-3424/OBI-3424 (denoted by 3424) is a novel prodrug bis-alkylating agent activated by AKR1C3. AKR1C3 is overexpressed in many types of cancer, particularly in liver, non-small cell lung, gastric, renal and CRPC cancer. Currently 3424 is being studied in phase 1/2 clinical trials for the treatment of solid and hematologic cancers, and it represents potentially a novel, selective anti-cancer agent for multiple indications. In this study, AKR1C3-dependent activation of 3424 was investigated in vitro using recombinant human AKR1C3. AKR1C3-dependent cytotoxicity of 3424 was determined in a wide range of human cancer cell lines with different AKR1C3 expression levels. In addition, anti-tumor activity of 3424 was also investigated in a broad panel of CDX and PDX models. AKR1C3-dependent activation of prodrug 3424 was evident by monitoring the decrease of 3424 and generation of the active form, 2660. Kinetic analysis indicated that AKR1C3 exhibited higher catalytic efficiency towards 3424 compared to the physiological substrates. There was a strong correlation between 3424 cytotoxic potency and AKR1C3 expression. The racemic mixture induced DNA cross-linking in a concentration dependent manner. Tumor growth inhibition of 3424 was shown to be better than or comparable to the standard of care chemotherapy at clinically achievable doses as a single agent in various CDX models with high expression of AKR1C3, including liver HepG2, lung H460, castration-resistant prostate VCaP, gastric SNU-16, and kidney A498 cancer cell lines. The excellent anti-tumor efficacy of 3424 was further demonstrated in PDX models which have high level of AKR1C3 expression, but not in a model with low level of AKR1C3 expression. In the combination therapy, we showed that 3424 could enhance the efficacy of the standard care of chemotherapy in the CDX models. The results described here highlight that 3424 exhibits AKR1C3-dependent cytotoxicity in vitro and anti-tumor activity in vivo in a wide range of human cancer types, which support further development of 3424 as an anti-cancer agent for treating different types of cancers and the use of AKR1C3 as a biomarker to profile cancer patients and further guide patient selection for therapy with 3424.
Keywords: AKR1C3; alkylating agent; cancers and anti-cancer agent; prodrug.
AJCR Copyright © 2021.
Conflict of interest statement
All authors are current employees paid by either Ascentawits Pharmaceuticals, LTD or OBI Pharma, Inc.
Figures


References
-
- Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3 alpha-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3 alpha/17 beta-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. - PubMed
-
- Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–574. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
