Context-Dependent Roles of Claudins in Tumorigenesis
- PMID: 34354941
- PMCID: PMC8329526
- DOI: 10.3389/fonc.2021.676781
Context-Dependent Roles of Claudins in Tumorigenesis
Abstract
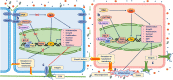
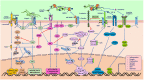
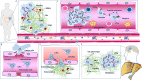
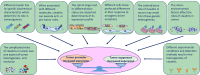
The barrier and fence functions of the claudin protein family are fundamental to tissue integrity and human health. Increasing evidence has linked claudins to signal transduction and tumorigenesis. The expression of claudins is frequently dysregulated in the context of neoplastic transformation. Studies have uncovered that claudins engage in nearly all aspects of tumor biology and steps of tumor development, suggesting their promise as targets for treatment or biomarkers for diagnosis and prognosis. However, claudins can be either tumor promoters or tumor suppressors depending on the context, which emphasizes the importance of taking various factors, including organ type, environmental context and genetic confounders, into account when studying the biological functions and targeting of claudins in cancer. This review discusses the complicated roles and intrinsic and extrinsic determinants of the context-specific effects of claudins in cancer.
Keywords: cancer; claudin; metastasis; tight junction; tumor heterogeneity; tumorigenesis.
Copyright © 2021 Li.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

