Adhesive Tissue Engineered Scaffolds: Mechanisms and Applications
- PMID: 34354985
- PMCID: PMC8329531
- DOI: 10.3389/fbioe.2021.683079
Adhesive Tissue Engineered Scaffolds: Mechanisms and Applications
Abstract
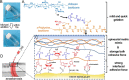
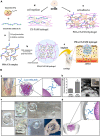
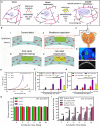
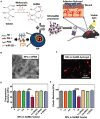
A variety of suture and bioglue techniques are conventionally used to secure engineered scaffold systems onto the target tissues. These techniques, however, confront several obstacles including secondary damages, cytotoxicity, insufficient adhesion strength, improper degradation rate, and possible allergic reactions. Adhesive tissue engineering scaffolds (ATESs) can circumvent these limitations by introducing their intrinsic tissue adhesion ability. This article highlights the significance of ATESs, reviews their key characteristics and requirements, and explores various mechanisms of action to secure the scaffold onto the tissue. We discuss the current applications of advanced ATES products in various fields of tissue engineering, together with some of the key challenges for each specific field. Strategies for qualitative and quantitative assessment of adhesive properties of scaffolds are presented. Furthermore, we highlight the future prospective in the development of advanced ATES systems for regenerative medicine therapies.
Keywords: adhesive tissue engineering scaffold; bone regeneration; cardiac regeneration; cartilage regeneration; nerve regeneration; scaffold; tissue regeneration; wound repair.
Copyright © 2021 Chen, Gil, Ning, Jin, Perez, Kabboul, Tomov and Serpooshan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ahearne M., Fernández-Pérez J., Masterton S., Madden P. W., Bhattacharjee P. (2020). Designing scaffolds for corneal regeneration. Adv. Funct. Mater. 30:1908996. 10.1002/adfm.201908996 - DOI
-
- Bahm J., Esser T., Sellhaus B., El-Kazzi W., Schuind F. (2018). Tension in Peripheral Nerve Suture. London: IntechOpen. 10.5772/intechopen.78722 - DOI
-
- Bai S., Zhang X., Lv X., Zhang M., Huang X., Shi Y., et al. (2020). Bioinspired mineral-organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv. Funct. Mater. 30:1908381. 10.1002/adfm.201908381 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

