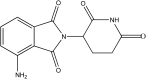
Inclusion complexation of the anticancer drug pomalidomide with cyclodextrins: fast dissolution and improved solubility
- PMID: 34355087
- PMCID: PMC8321930
- DOI: 10.1016/j.heliyon.2021.e07581
Inclusion complexation of the anticancer drug pomalidomide with cyclodextrins: fast dissolution and improved solubility
Abstract
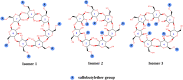
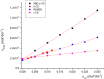
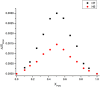
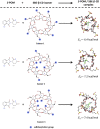
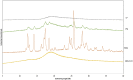
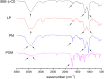
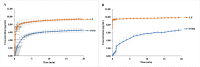
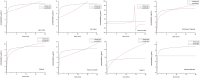
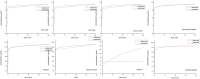
Pomalidomide (POM), a potent anticancer thalidomide analogue was characterized in terms of cyclodextrin complexation to improve its aqueous solubility and maintain its anti-angiogenic activity. The most promising cyclodextrin derivatives were selected by phase-solubility studies. From the investigated nine cyclodextrins - differing in cavity size, nature of substituents, degree of substitution and charge - the highest solubility increase was observed with sulfobutylether-β-cyclodextrin (SBE-β-CD). The inclusion complexation between POM and SBE-β-CD was further characterized with a wide variety of state-of-the-art analytical techniques, such as nuclear magnetic resonance spectroscopy (NMR), infrared spectroscopy (IR), circular dichroism spectroscopy, fluorescence spectroscopy as well as X-ray powder diffraction method (XRD). Job plot titration by NMR and the AL-type phase-solubility diagram indicated 1:1 stoichiometry in a liquid state. Complementary analytical methods were employed for the determination of the stability constant of the complex; the advantages and disadvantages of the different approaches are also discussed. Inclusion complex formation was also assessed by molecular modelling study. Solid state complexation in a 1:1 M ratio was carried out by lyophilization and investigated by IR and XRD. The complex exhibited fast-dissolution with immediate release of POM, when compared to the pure drug at acidic and neutral pH. Kinetic analysis of POM release from lyophilized complex shows that Korsmeyer-Peppas and Weibull model described the best the dissolution kinetics. The cytotoxicity of the complex was tested against the LP-1 human myeloma cell line which revealed that supramolecular interactions did not significantly affect the anti-cancer activity of the drug. Overall, our results suggest that the inclusion complexation of POM with SBE-β-CD could be a promising approach for developing more effective POM formulations with increased solubility.
Keywords: Comparative dissolution; Cyclodextrin complexation; Inclusion complex; Pomalidomide; Pomalyst®; Solubility.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ramasamy K., Gay F., Weisel K., Zweegman S., Mateos M.V., Richardson P. Improving outcomes for patients with relapsed multiple myeloma: challenges and considerations of current and emerging treatment options. Blood Rev. 2021:100808. - PubMed
-
- Dimopoulos M., Weisel K., Moreau P., Anderson L.D., White D., San-Miguel J., Sonneveld P., Engelhardt M., Jenner M., Corso A., Dürig J., Pavic M., Salomo M., Casal E., Srinivasan S., Yu X., Nguyen T.V., Biyukov T., Peluso T., Richardson P. 2020. Pomalidomide, Bortezomib, and Dexamethasone for Multiple Myeloma Previously Treated with Lenalidomide (OPTIMISMM): Outcomes by Prior Treatment at First Relapse, Leukemia. - PMC - PubMed
-
- EMA assesment report. 2021. https://www.ema.europa.eu/en/documents/assessment-report/pomalidomide-ce...
-
- Kanaujia P., Poovizhi P., Ng W.K., Tan R.B.H. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015;285:2–15.
-
- Carrier R.L., Miller L.A., Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J. Contr. Release. 2007;123:78–99. - PubMed
LinkOut - more resources
Full Text Sources

