Recent advances in the development of covalent inhibitors
- PMID: 34355176
- PMCID: PMC8292994
- DOI: 10.1039/d1md00068c
Recent advances in the development of covalent inhibitors
Abstract
The use of covalent inhibitors in the field of drug discovery has attracted considerable attention in the 2000s. As a result, more than 50 covalent drugs are currently on the market, and numerous covalent drug candidates are now under development. Therefore, interest in covalent drugs is expected to continue in the future. The purpose of this focused review is to provide an understanding of the development of covalent inhibitors by describing their inherent characteristics, possibilities, and limitations based on their mechanistic differences from noncovalent inhibitors. We also introduce the latest covalent warheads that can be applied to the development of potential covalent inhibitors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


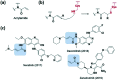
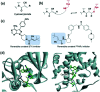
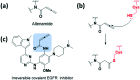


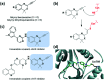

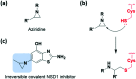
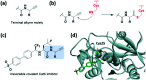
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

