Recent advances in urea- and thiourea-containing compounds: focus on innovative approaches in medicinal chemistry and organic synthesis
- PMID: 34355177
- PMCID: PMC8293013
- DOI: 10.1039/d1md00058f
Recent advances in urea- and thiourea-containing compounds: focus on innovative approaches in medicinal chemistry and organic synthesis
Abstract
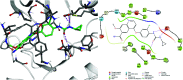
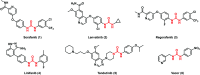
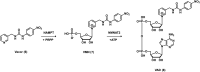
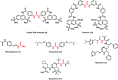
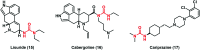
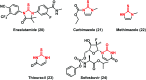
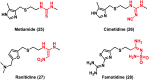
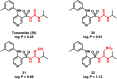
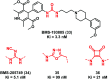
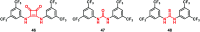
Urea and thiourea represent privileged structures in medicinal chemistry. Indeed, these moieties constitute a common framework of a variety of drugs and bioactive compounds endowed with a broad range of therapeutic and pharmacological properties. Herein, we provide an overview of the state-of-the-art of urea and thiourea-containing pharmaceuticals. We also review the diverse approaches pursued for (thio)urea bioisosteric replacements in medicinal chemistry applications. Finally, representative examples of recent advances in the synthesis of urea- and thiourea-based compounds by enabling chemical tools are discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
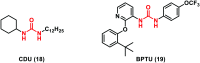

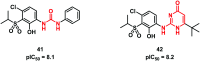


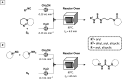
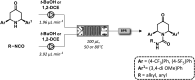
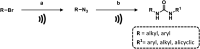

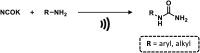
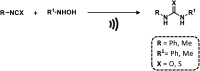
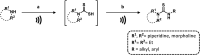
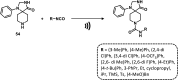
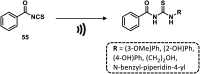
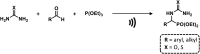
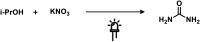
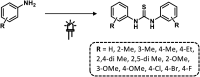
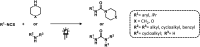
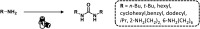
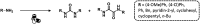


References
-
- Garuti L. Roberti M. Bottegoni G. Ferraro M. Curr. Med. Chem. 2016;23:1528–1548. - PubMed
-
- Shimshoni J. A. Bialer M. Wlodarczyk B. Finnell R. H. Yagen B. J. Med. Chem. 2007;50:6419–6427. - PubMed
-
- Keche A. P. Hatnapure G. D. Tale R. H. Rodge A. H. Birajdar S. S. Kamble V. M. Bioorg. Med. Chem. Lett. 2012;22:3445–3448. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

