How we('ll) treat paroxysmal nocturnal haemoglobinuria: diving into the future
- PMID: 34355382
- PMCID: PMC9291300
- DOI: 10.1111/bjh.17753
How we('ll) treat paroxysmal nocturnal haemoglobinuria: diving into the future
Abstract
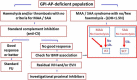
Paroxysmal nocturnal haemoglobinuria (PNH) is characterized by complement-mediated intravascular haemolysis, severe thrombophilia and bone marrow failure. While for patients with bone marrow failure the treatment follows that of immune-mediated aplastic anaemia, that of classic, haemolytic PNH is based on anti-complement medication. The anti-C5 monoclonal antibody eculizumab has revolutionized treatment, resulting in control of intravascular haemolysis and thromboembolic risk, with improved long-term survival. Novel strategies of complement inhibition are emerging. New anti-C5 agents reproduce the safety and efficacy of eculizumab, with improved patient convenience. Proximal complement inhibitors have been developed to address C3-mediated extra-vascular haemolysis and seem to improve haematological response.
Keywords: extravascular haemolysis; intravascular haemolysis; paroxysmal nocturnal haemoglobinuria; proximal complement inhibitors; terminal complement inhibitors.
© 2021 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
AMR has received research support from Alexion, Novartis, Alnylam and Rapharma, lecture fees from Alexion, Novartis, Pfizer and Apellis, served as a member of the advisory/investigator board for Alexion, Roche, Achillion, Novartis, Apellis, Biocryst and Samsung, and served as consultant for Amyndas. RPdL has received research funding from Alexion, Amgen, Jazz Pharmaceuticals and Pfizer; consulted for and received honoraria from Alexion, Amgen, Gilead, Jazz Pharmaceuticals, Keocyte, MSD, Novartis, Pfizer, Roche, Samsung and Mallinckrodt.
Figures


Comment in
-
Lactate dehydrogenase versus haemoglobin: which one is the better marker in paroxysmal nocturnal haemoglobinuria?Br J Haematol. 2022 Jan;196(2):264-265. doi: 10.1111/bjh.17860. Epub 2021 Dec 19. Br J Haematol. 2022. PMID: 34923628 No abstract available.
References
-
- Risitano AM. Paroxysmal nocturnal hemoglobinuria. In: Silverberg DS, editor. Anemia. Rijeka: InTech; 2012. p. 331–74.
-
- Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG‐A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73(4):703–11. - PubMed
-
- Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, et al. Abnormalities of PIG‐A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330(4):249–55. - PubMed
-
- Rotoli B, Luzzatto L. Paroxysmal nocturnal haemoglobinuria. Bailliere's Clin Haematol. 1989;2(1):113–38. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

