Naa12 compensates for Naa10 in mice in the amino-terminal acetylation pathway
- PMID: 34355692
- PMCID: PMC8376253
- DOI: 10.7554/eLife.65952
Naa12 compensates for Naa10 in mice in the amino-terminal acetylation pathway
Abstract
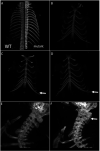
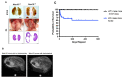
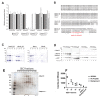
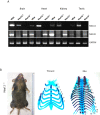
Amino-terminal acetylation is catalyzed by a set of N-terminal acetyltransferases (NATs). The NatA complex (including X-linked Naa10 and Naa15) is the major acetyltransferase, with 40-50% of all mammalian proteins being potential substrates. However, the overall role of amino-terminal acetylation on a whole-organism level is poorly understood, particularly in mammals. Male mice lacking Naa10 show no globally apparent in vivo amino-terminal acetylation impairment and do not exhibit complete embryonic lethality. Rather Naa10 nulls display increased neonatal lethality, and the majority of surviving undersized mutants exhibit a combination of hydrocephaly, cardiac defects, homeotic anterior transformation, piebaldism, and urogenital anomalies. Naa12 is a previously unannotated Naa10-like paralog with NAT activity that genetically compensates for Naa10. Mice deficient for Naa12 have no apparent phenotype, whereas mice deficient for Naa10 and Naa12 display embryonic lethality. The discovery of Naa12 adds to the currently known machinery involved in amino-terminal acetylation in mice.
Keywords: N-terminal acetylation; NAA10; NAA12; developmental biology; embryonic lethality; hydrocephaly; mouse; protein modification.
© 2021, Kweon et al.
Conflict of interest statement
HK, ML, MD, SS, LG, TP, NM, RR, DB, AG, MF, JC, AS, SL, AI, EM, SS, SJ, SJ, SJ, SC, TK, HK, CL, TR, TA, RM, GO, GL No competing interests declared
Figures




Similar articles
-
Evaluating possible maternal effect lethality and genetic background effects in Naa10 knockout mice.PLoS One. 2024 May 7;19(5):e0301328. doi: 10.1371/journal.pone.0301328. eCollection 2024. PLoS One. 2024. PMID: 38713657 Free PMC article.
-
A novel NAA10 p.(R83H) variant with impaired acetyltransferase activity identified in two boys with ID and microcephaly.BMC Med Genet. 2019 Jun 7;20(1):101. doi: 10.1186/s12881-019-0803-1. BMC Med Genet. 2019. PMID: 31174490 Free PMC article.
-
NAA10 p.(D10G) and NAA10 p.(L11R) Variants Hamper Formation of the NatA N-Terminal Acetyltransferase Complex.Int J Mol Sci. 2020 Nov 26;21(23):8973. doi: 10.3390/ijms21238973. Int J Mol Sci. 2020. PMID: 33255974 Free PMC article.
-
N-α-acetyltransferase 10 (NAA10) in development: the role of NAA10.Exp Mol Med. 2018 Jul 27;50(7):1-11. doi: 10.1038/s12276-018-0105-2. Exp Mol Med. 2018. PMID: 30054454 Free PMC article. Review.
-
NAA10-related syndrome.Exp Mol Med. 2018 Jul 27;50(7):1-10. doi: 10.1038/s12276-018-0098-x. Exp Mol Med. 2018. PMID: 30054457 Free PMC article. Review.
Cited by
-
NSUN2 lactylation drives cancer cell resistance to ferroptosis through enhancing GCLC-dependent glutathione synthesis.Redox Biol. 2025 Feb;79:103479. doi: 10.1016/j.redox.2024.103479. Epub 2024 Dec 19. Redox Biol. 2025. PMID: 39742570 Free PMC article.
-
Illuminating the impact of N-terminal acetylation: from protein to physiology.Nat Commun. 2025 Jan 15;16(1):703. doi: 10.1038/s41467-025-55960-5. Nat Commun. 2025. PMID: 39814713 Free PMC article. Review.
-
Evaluating possible maternal effect lethality and genetic background effects in Naa10 knockout mice.bioRxiv [Preprint]. 2024 Jan 10:2023.04.27.538618. doi: 10.1101/2023.04.27.538618. bioRxiv. 2024. Update in: PLoS One. 2024 May 7;19(5):e0301328. doi: 10.1371/journal.pone.0301328. PMID: 37163119 Free PMC article. Updated. Preprint.
-
Phenotypic variability and gastrointestinal manifestations/interventions for growth in NAA10-related neurodevelopmental syndrome.Am J Med Genet A. 2023 May;191(5):1293-1300. doi: 10.1002/ajmg.a.63152. Epub 2023 Feb 21. Am J Med Genet A. 2023. PMID: 36810866 Free PMC article.
-
NAA10 (N-Alpha-Acetyltransferase 10): A Multifunctional Regulator in Development, Disease, and Cancer.Cells. 2025 Jun 7;14(12):863. doi: 10.3390/cells14120863. Cells. 2025. PMID: 40558489 Free PMC article. Review.
References
-
- Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. PNAS. 2009;106:8157–8162. doi: 10.1073/pnas.0901931106. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

