The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs
- PMID: 34355693
- PMCID: PMC8346280
- DOI: 10.7554/eLife.65655
The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs
Abstract
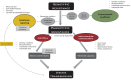
Monitoring local mosquito populations for insecticide resistance is critical for effective vector-borne disease control. However, widely used phenotypic assays, which are designed to monitor the emergence and spread of insecticide resistance (technical resistance), do not translate well to the efficacy of vector control products to suppress mosquito numbers in the field (practical resistance). This is because standard testing conditions such as environmental conditions, exposure dose, and type of substrate differ dramatically from those experienced by mosquitoes under field conditions. In addition, field mosquitoes have considerably different physiological characteristics such as age and blood-feeding status. Beyond this, indirect impacts of insecticide resistance and/or exposure on mosquito longevity, pathogen development, host-seeking behavior, and blood-feeding success impact disease transmission. Given the limited number of active ingredients currently available and the observed discordance between resistance and disease transmission, we conclude that additional testing guidelines are needed to determine practical resistance-the efficacy of vector control tools under relevant local conditions- in order to obtain programmatic impact.
Keywords: Aedes; Anopheles; Insecticide resistance; epidemiology; evolutionary biology; global health; policy; surveillance; vector control.
© 2021, Namias et al.
Conflict of interest statement
AN, NJ, KP, SH No competing interests declared
Figures
References
-
- Aïzoun N, Ossè R, Azondekon R, Alia R, Oussou O, Gnanguenon V, Aikpon R, Padonou GG, Akogbéto M. Comparison of the standard WHO susceptibility tests and the CDC bottle bioassay for the determination of insecticide susceptibility in malaria vectors and their correlation with biochemical and molecular biology assays in Benin, west africa. Parasites & Vectors. 2013;6:147. doi: 10.1186/1756-3305-6-147. - DOI - PMC - PubMed
-
- Allossogbe M, Gnanguenon V, Yovogan B, Akinro B, Anagonou R, Agossa F, Houtoukpe A, Padonou GG, Akogbeto M. WHO cone bio-assays of classical and new-generation long-lasting insecticidal nets call for innovative insecticides targeting the knock-down resistance mechanism in Benin. Malaria Journal. 2017;16:77. doi: 10.1186/s12936-017-1727-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

