Unexpected plasticity in the life cycle of Trypanosoma brucei
- PMID: 34355698
- PMCID: PMC8448533
- DOI: 10.7554/eLife.66028
Unexpected plasticity in the life cycle of Trypanosoma brucei
Abstract
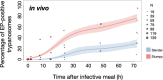
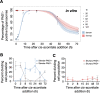
African trypanosomes cause sleeping sickness in humans and nagana in cattle. These unicellular parasites are transmitted by the bloodsucking tsetse fly. In the mammalian host's circulation, proliferating slender stage cells differentiate into cell cycle-arrested stumpy stage cells when they reach high population densities. This stage transition is thought to fulfil two main functions: first, it auto-regulates the parasite load in the host; second, the stumpy stage is regarded as the only stage capable of successful vector transmission. Here, we show that proliferating slender stage trypanosomes express the mRNA and protein of a known stumpy stage marker, complete the complex life cycle in the fly as successfully as the stumpy stage, and require only a single parasite for productive infection. These findings suggest a reassessment of the traditional view of the trypanosome life cycle. They may also provide a solution to a long-lasting paradox, namely the successful transmission of parasites in chronic infections, despite low parasitemia.
Keywords: development; infectious disease; life cycle; microbiology; sleeping sickness; transmission; trypanosoma; tsetse fly.
© 2021, Schuster et al.
Conflict of interest statement
SS, JL, IS, HZ, CR, TM, BM, ME No competing interests declared
Figures




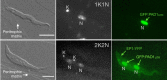






 ) slender forms in the blood, while disseminating into the interstitium and various tissues, including fat and brain. At least two triggers (SIF or ES) launch the PAD1-dependent differentiation pathway (light green boxes) to the cell cycle-arrested (
) slender forms in the blood, while disseminating into the interstitium and various tissues, including fat and brain. At least two triggers (SIF or ES) launch the PAD1-dependent differentiation pathway (light green boxes) to the cell cycle-arrested ( ) stumpy bloodstream stage. Stumpy trypanosomes can establish a fly infection when taken up with the bloodmeal of a tsetse. The work described here reveals that proliferating slender stage trypanosomes are equally effective for tsetse transmission, that a single parasite suffices, and that the population can continuously divide while differentiating to the procyclic insect stage. The triggers that initiate further developmental transitions are temperature (°C), cis-aconitate (CA) and glucose deprivation (↓Glc).
) stumpy bloodstream stage. Stumpy trypanosomes can establish a fly infection when taken up with the bloodmeal of a tsetse. The work described here reveals that proliferating slender stage trypanosomes are equally effective for tsetse transmission, that a single parasite suffices, and that the population can continuously divide while differentiating to the procyclic insect stage. The triggers that initiate further developmental transitions are temperature (°C), cis-aconitate (CA) and glucose deprivation (↓Glc).Comment in
-
A two-stage solution.Elife. 2021 Sep 17;10:e72980. doi: 10.7554/eLife.72980. Elife. 2021. PMID: 34534076 Free PMC article.
-
Response to comment on 'Unexpected plasticity in the life cycle of Trypanosoma Brucei'.Elife. 2022 Feb 1;11:e75922. doi: 10.7554/eLife.75922. Elife. 2022. PMID: 35103593 Free PMC article.
-
Comment on 'Unexpected plasticity in the life cycle of Trypanosoma brucei'.Elife. 2022 Feb 1;11:e74985. doi: 10.7554/eLife.74985. Elife. 2022. PMID: 35103595 Free PMC article.
References
-
- Alvar J, Alves F, Bucheton B, Burrows L, Büscher P, Carrillo E, Felger I, Hübner MP, Moreno J, Pinazo MJ, Ribeiro I, Sosa-Estani S, Specht S, Tarral A, Wourgaft NS, Bilbe G. Implications of asymptomatic infection for the natural history of selected parasitic tropical diseases. Seminars in immunopathology. 2020;42:231–246. doi: 10.1007/s00281-020-00796-y. - DOI - PMC - PubMed
-
- Baker JR, Robertson DH. An experiment on the infectivity to Glossina morsitans of a strain of Trypanosoma rhodesiense and of a strain of T. brucei, with some observations on the longevity of infected flies. Annals of tropical medicine and parasitology. 1957;51:121–135. doi: 10.1080/00034983.1957.11685801. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

