Optic disc edema and chorioretinal folds develop during strict 6° head-down tilt bed rest with or without artificial gravity
- PMID: 34355874
- PMCID: PMC8343460
- DOI: 10.14814/phy2.14977
Optic disc edema and chorioretinal folds develop during strict 6° head-down tilt bed rest with or without artificial gravity
Abstract
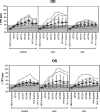
Spaceflight associated neuro-ocular syndrome (SANS) is hypothesized to develop as a consequence of the chronic headward fluid shift that occurs in sustained weightlessness. We exposed healthy subjects (n = 24) to strict 6° head-down tilt bed rest (HDTBR), an analog of weightlessness that generates a sustained headward fluid shift, and we monitored for ocular changes similar to findings that develop in SANS. Two-thirds of the subjects received a daily 30-min exposure to artificial gravity (AG, 1 g at center of mass, ~0.3 g at eye level) during HDTBR by either continuous (cAG, n = 8) or intermittent (iAG, n = 8) short-arm centrifugation to investigate whether this intervention would attenuate headward fluid shift-induced ocular changes. Optical coherence tomography images were acquired to quantify changes in peripapillary total retinal thickness (TRT), retinal nerve fiber layer thickness, and choroidal thickness, and to detect chorioretinal folds. Intraocular pressure (IOP), optical biometry, and standard automated perimetry data were collected. TRT increased by 35.9 µm (95% CI, 19.9-51.9 µm, p < 0.0001), 36.5 µm (95% CI, 4.7-68.2 µm, p = 0.01), and 27.6 µm (95% CI, 8.8-46.3 µm, p = 0.0005) at HDTBR day 58 in the control, cAG, and iAG groups, respectively. Chorioretinal folds developed in six subjects across the groups, despite small increases in IOP. Visual function outcomes did not change. These findings validate strict HDTBR without elevated ambient CO2 as a model for investigating SANS and suggest that a fluid shift reversal of longer duration and/or greater magnitude at the eye may be required to prevent or mitigate SANS.
Keywords: artificial gravity; bed rest; centrifugation; chorioretinal folds; retinal thickness; spaceflight analog; spaceflight associated neuro-ocular syndrome.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Balasubramanian, S. , Tepelus, T. , Stenger, M. B. , Lee, S. M. C. , Laurie, S. S. , Liu, J. H. K. , Feiveson, A. H. , Sadda, S. R. , Huang, A. S. , & Macias, B. R. (2018). Thigh cuffs as a countermeasure for ocular changes in simulated weightlessness. Ophthalmology, 125, 459–460. 10.1016/j.ophtha.2017.10.023 - DOI - PubMed
-
- Draeger, J. , Schwartz, R. , Groenhoff, S. , & Stern, C. (1995). Self‐tonometry under microgravity conditions. Aviation, Space and Environmental Medicine, 66, 568–570. - PubMed
-
- Fannin, L. A. , Schiffman, J. C. , & Budenz, D. L. (2003). Risk factors for hypotony maculopathy. Ophthalmology, 110, 1185–1191. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

