Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C-H Bonds Elaboration
- PMID: 34355884
- PMCID: PMC8796199
- DOI: 10.1021/acs.chemrev.1c00263
Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C-H Bonds Elaboration
Abstract
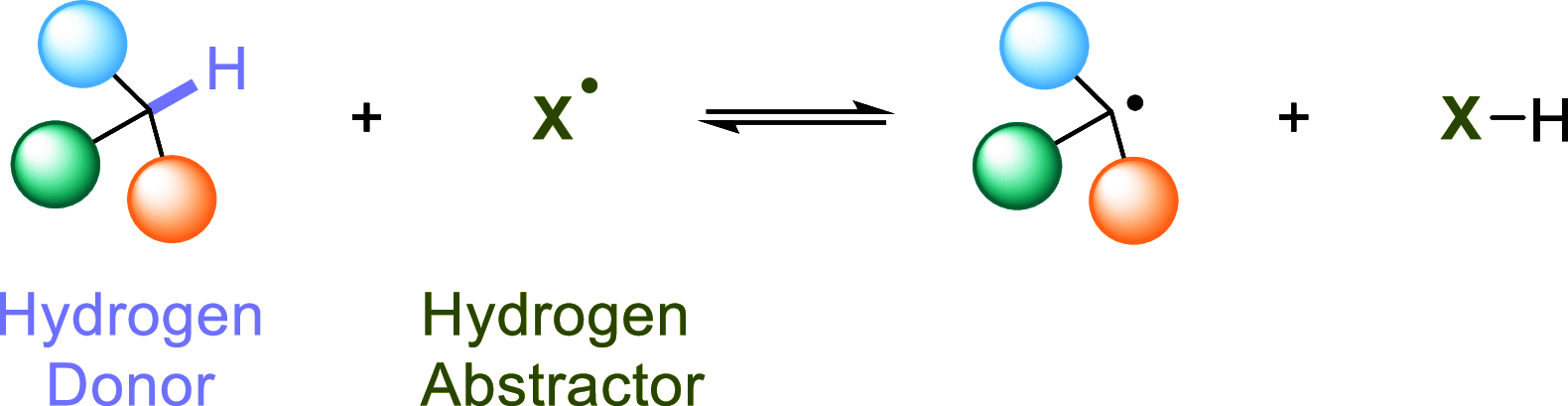
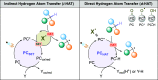
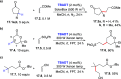
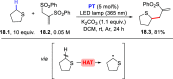
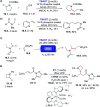
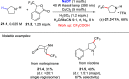
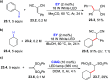
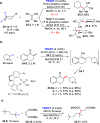
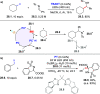
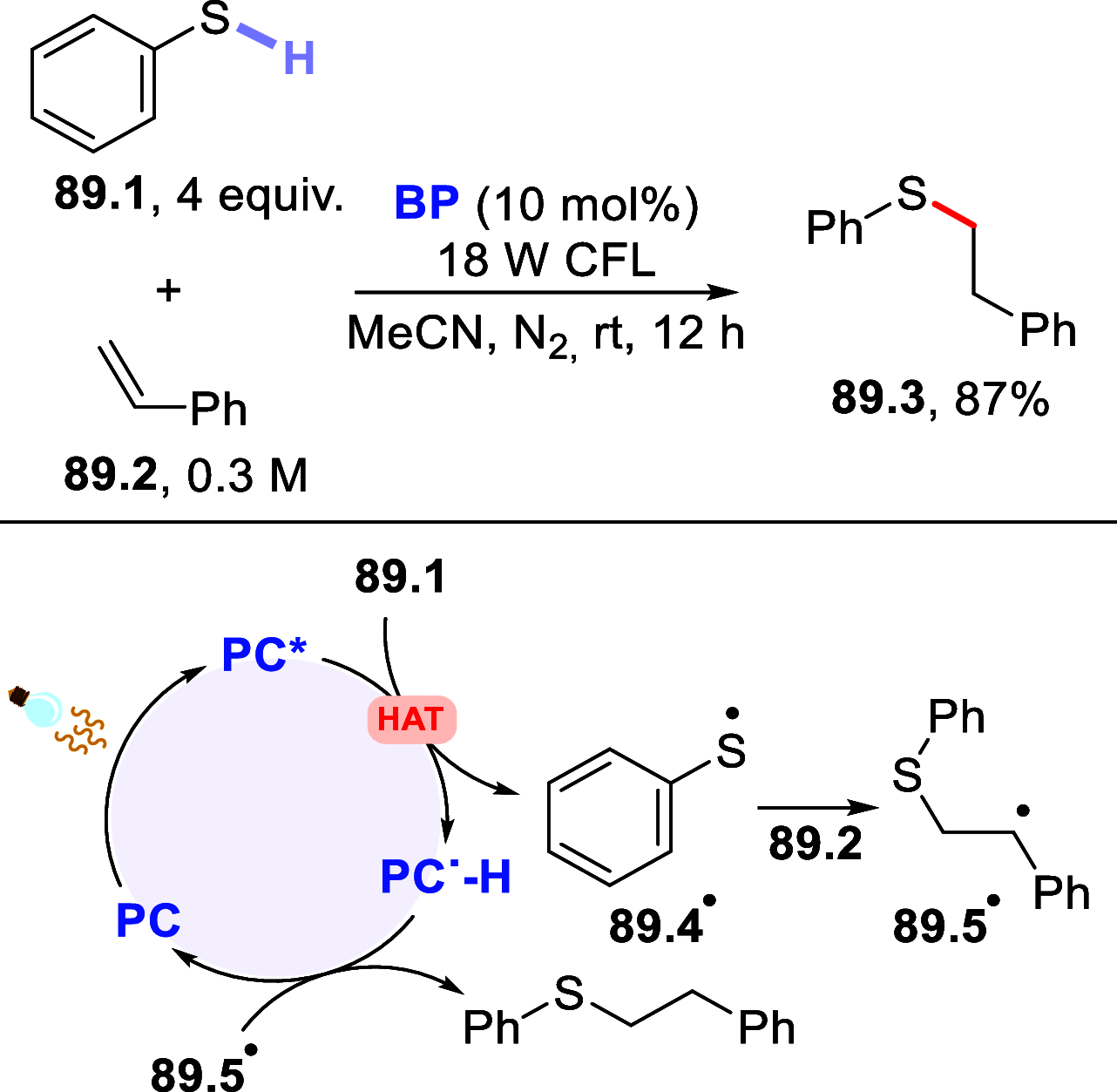
Direct photocatalyzed hydrogen atom transfer (d-HAT) can be considered a method of choice for the elaboration of aliphatic C-H bonds. In this manifold, a photocatalyst (PCHAT) exploits the energy of a photon to trigger the homolytic cleavage of such bonds in organic compounds. Selective C-H bond elaboration may be achieved by a judicious choice of the hydrogen abstractor (key parameters are the electronic character and the molecular structure), as well as reaction additives. Different are the classes of PCsHAT available, including aromatic ketones, xanthene dyes (Eosin Y), polyoxometalates, uranyl salts, a metal-oxo porphyrin and a tris(amino)cyclopropenium radical dication. The processes (mainly C-C bond formation) are in most cases carried out under mild conditions with the help of visible light. The aim of this review is to offer a comprehensive survey of the synthetic applications of photocatalyzed d-HAT.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




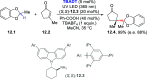
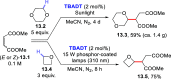
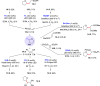
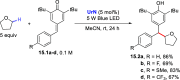

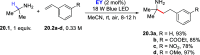




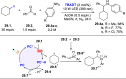
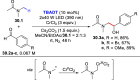

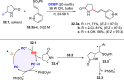
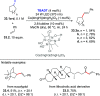













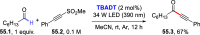
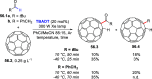
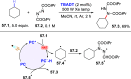

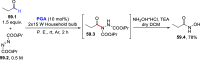
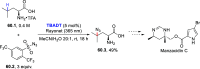
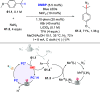



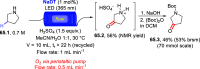
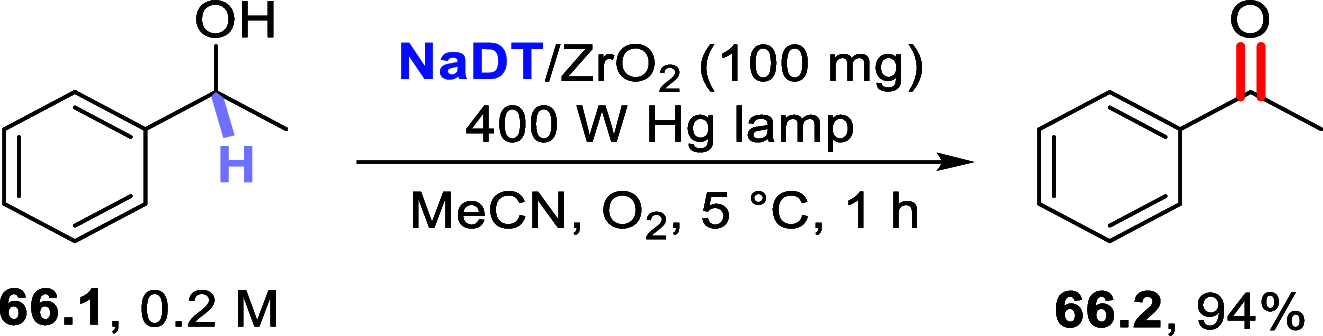

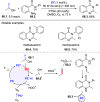


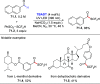
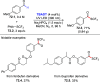
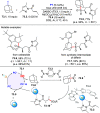

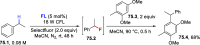

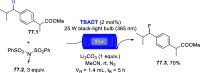

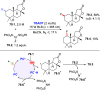

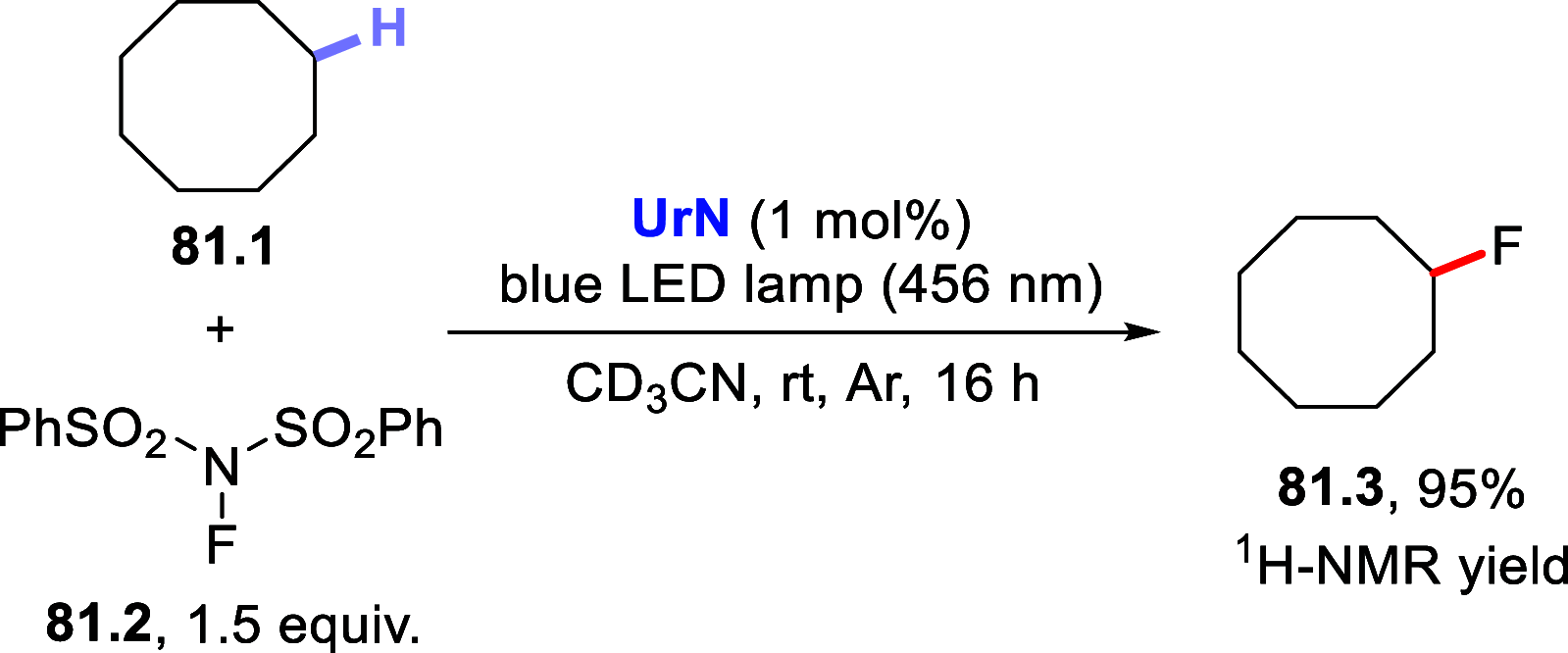
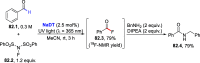
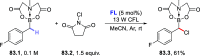

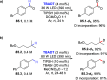
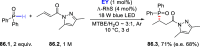



References
-
- Braun M.Modern Enolate Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015.
-
- Stolz D.; Kazmaier U.. Metal Enolates as Synthons in Organic Chemistry. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd: Chichester, UK, 2010.
-
- Goldman A. S.; Goldberg K. I.. Organometallic C–H Bond Activation: An Introduction. In Activation and Functionalization of C–H Bonds; ACS Symposium Series 2004; Vol. 885, pp 1–43.
-
- Sambiagio C.; Schönbauer D.; Blieck R.; Dao-Huy T.; Pototschnig G.; Schaaf P.; Wiesinger T.; Zia M. F.; Wencel-Delord J.; Besset T.; Maes B. U. W.; Schnürch M. A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. 10.1039/C8CS00201K. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

