Role and Evolution of the Extracellular Matrix in the Acquisition of Complex Multicellularity in Eukaryotes: A Macroalgal Perspective
- PMID: 34356075
- PMCID: PMC8307928
- DOI: 10.3390/genes12071059
Role and Evolution of the Extracellular Matrix in the Acquisition of Complex Multicellularity in Eukaryotes: A Macroalgal Perspective
Abstract
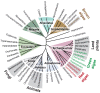
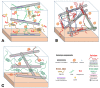
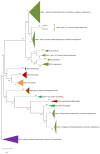
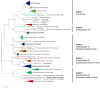
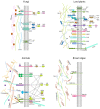
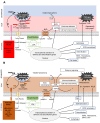
Multicellular eukaryotes are characterized by an expanded extracellular matrix (ECM) with a diversified composition. The ECM is involved in determining tissue texture, screening cells from the outside medium, development, and innate immunity, all of which are essential features in the biology of multicellular eukaryotes. This review addresses the origin and evolution of the ECM, with a focus on multicellular marine algae. We show that in these lineages the expansion of extracellular matrix played a major role in the acquisition of complex multicellularity through its capacity to connect, position, shield, and defend the cells. Multiple innovations were necessary during these evolutionary processes, leading to striking convergences in the structures and functions of the ECMs of algae, animals, and plants.
Keywords: biomechanical properties; cell wall; development; evolutionary convergences; extracellular matrix; hydric and salinity stress; innate immunity; marine macroalgae; multicellularity; sulfated polysaccharides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Grosberg R.K., Strathmann R.R. The evolution of multicellularity: A minor major transition? Annu. Rev. Ecol. Evol. Syst. 2007;38:621–654. doi: 10.1146/annurev.ecolsys.36.102403.114735. - DOI
-
- Cock J.M., Collen J. Independent emergence of complex multicellularity in the brown and red algae. In: Ruiz-Trillo I., Nedelcu A.M., editors. Evolutionary Transitions to Multicellular Life. Springer; Dordrecht, The Netherlands: 2015. pp. 335–361.
-
- Michel G., Tonon T., Scornet D., Cock J.M., Kloareg B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus Siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010;188:82–97. doi: 10.1111/j.1469-8137.2010.03374.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

