Alternative RNA Splicing-The Trojan Horse of Cancer Cells in Chemotherapy
- PMID: 34356101
- PMCID: PMC8306420
- DOI: 10.3390/genes12071085
Alternative RNA Splicing-The Trojan Horse of Cancer Cells in Chemotherapy
Abstract
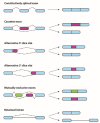
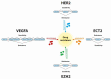
Almost all transcribed human genes undergo alternative RNA splicing, which increases the diversity of the coding and non-coding cellular landscape. The resultant gene products might have distinctly different and, in some cases, even opposite functions. Therefore, the abnormal regulation of alternative splicing plays a crucial role in malignant transformation, development, and progression, a fact supported by the distinct splicing profiles identified in both healthy and tumor cells. Drug resistance, resulting in treatment failure, still remains a major challenge for current cancer therapy. Furthermore, tumor cells often take advantage of aberrant RNA splicing to overcome the toxicity of the administered chemotherapeutic agents. Thus, deciphering the alternative RNA splicing variants in tumor cells would provide opportunities for designing novel therapeutics combating cancer more efficiently. In the present review, we provide a comprehensive outline of the recent findings in alternative splicing in the most common neoplasms, including lung, breast, prostate, head and neck, glioma, colon, and blood malignancies. Molecular mechanisms developed by cancer cells to promote oncogenesis as well as to evade anticancer drug treatment and the subsequent chemotherapy failure are also discussed. Taken together, these findings offer novel opportunities for future studies and the development of targeted therapy for cancer-specific splicing variants.
Keywords: alternative splicing; cancer pathobiology; drug resistance; splice variants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

