Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency
- PMID: 34356339
- PMCID: PMC8301024
- DOI: 10.3390/antiox10071106
Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency
Abstract
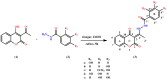
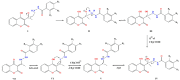
Compounds from the plant world that possess antioxidant abilities are of special importance for the food and pharmaceutical industry. Coumarins are a large, widely distributed group of natural compounds, usually found in plants, often with good antioxidant capacity. The coumarin-hydroxybenzohydrazide derivatives were synthesized using a green, one-pot protocol. This procedure includes the use of an environmentally benign mixture (vinegar and ethanol) as a catalyst and solvent, as well as very easy isolation of the desired products. The obtained compounds were structurally characterized by IR and NMR spectroscopy. The purity of all compounds was determined by HPLC and by elemental microanalysis. In addition, these compounds were evaluated for their in vitro antioxidant activity. Mechanisms of antioxidative activity were theoretically investigated by the density functional theory approach and the calculated values of various thermodynamic parameters, such as bond dissociation enthalpy, proton affinity, frontier molecular orbitals, and ionization potential. In silico calculations indicated that hydrogen atom transfer and sequential proton loss-electron transfer reaction mechanisms are probable, in non-polar and polar solvents respectively. Additionally, it was found that the single-electron transfer followed by proton transfer was not an operative mechanism in either solvent. The conducted tests indicate the excellent antioxidant activity, as well as the low potential toxicity, of the investigated compounds, which makes them good candidates for potential use in food chemistry.
Keywords: DFT; antioxidants; coumarins; green synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sadeer N.B., Llorent-Martínez E.J., Bene K., Mahomoodally M.F., Mollica A., Sinan K.I., Zengin G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019;174:19–33. doi: 10.1016/j.jpba.2019.05.041. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

