Clinical and Biological Characteristics of Medullary and Extramedullary Plasma Cell Dyscrasias
- PMID: 34356484
- PMCID: PMC8301115
- DOI: 10.3390/biology10070629
Clinical and Biological Characteristics of Medullary and Extramedullary Plasma Cell Dyscrasias
Abstract
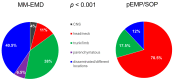
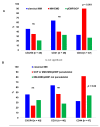
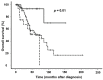
Background: Extramedullary plasma cell (PC) disorders may occur as extramedullary disease in multiple myeloma (MM-EMD) or as primary extramedullary plasmocytoma (pEMP)/solitary osseous plasmocytoma (SOP). In this study, we aimed to obtain insights into the molecular mechanisms of extramedullary spread of clonal PC. Methods: Clinical and biological characteristics of 87 patients with MM-EMD (n = 49), pEMP/SOP (n = 20) and classical MM (n = 18) were analyzed by using immunohistochemistry (CXCR4, CD31, CD44 and CD81 staining) and cytoplasmic immunoglobulin staining combined with fluorescence in situ hybridization (cIg-FISH). Results: High expression of CD44, a cell-surface glycoprotein involved in cell-cell interactions, was significantly enriched in MM-EMD (90%) vs. pEMP/SOP (27%) or classical MM (33%) (p < 0.001). In addition, 1q21 amplification by clonal PC occurred at a similar frequency of MM-EMD (33%), pEMP/SOP (57%) and classical MM (44%). Conversely, del(17p13), t(4;14) and t(14;16) were completely absent in pEMP/SOP. Besides this, 1q21 amplification was identified in 64% of not paraskeletal samples from MM-EMD or pEMP compared to 9% of SOP or paraskeletal MM-EMD/pEMP and 44% of classical MM samples, respectively (p = 0.02). Conclusion: Expression of molecules involved in homing and cytogenetic aberrations differ between MM with or without EMD and pEMP/SOP.
Keywords: cytogenetics; extramedullary; immunohistochemistry; multiple myeloma; plasma cell disorder.
Conflict of interest statement
S.J.: financial support of educational meetings by Celgene GmbH, Sobi GmbH, Bristol-Myers Squibb GmbH, AOP Orphan GmbH and Amgen GmbH. P.L.: none. A.N.: none. D.B.: none. L.R.: speaker honoraria from BMS/Celgene, Sanofi, GSK, Oncopeptides and Janssen. D.J.: none. C.B.: personal fees from Sanofi Aventis, Merck KgA, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly Imclone, Bayer Healthcare, GSO Contract Research, AOK Rheinland-Hamburg and Novartis. G.S.: none. I.W.B.: none. M.S.-H.: advisory role or expert testimony (no personal fees): Celgene GmbH, Kite/Pharma Gilead and Sanofi. Other financial relationships: financial support of educational meetings at the Carl-Thiem-Klinikum Cottbus gGmbH by Janssen-Cilag GmbH, Takeda Pharma Vertrieb GmbH & Co. KG, Novartis Pharma Oncology, Pfizer Pharma GmbH, Roche Pharma AG, Vifor Pharma.
Figures



References
-
- Caers J., Paiva B., Zamagni E., Leleu X., Bladé J., Kristinsson S.Y., Touzeau C., Abildgaard N., Terpos E., Heusschen R., et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J. Hematol. Oncol. 2018;11:10. doi: 10.1186/s13045-017-0549-1. - DOI - PMC - PubMed
-
- Usmani S.Z., Heuck C., Mitchell A., Szymonifka J., Nair B., Hoering A., Alsayed Y., Waheed S., Haider S., Restrepo A., et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97:1761–1767. doi: 10.3324/haematol.2012.065698. - DOI - PMC - PubMed
-
- Pour L., Sevcikova S., Greslikova H., Kupska R., Majkova P., Zahradova L., Sandecka V., Adam Z., Krejci M., Kuglik P., et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99:360–364. doi: 10.3324/haematol.2013.094409. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

