Osteopontin in Cardiovascular Diseases
- PMID: 34356671
- PMCID: PMC8301767
- DOI: 10.3390/biom11071047
Osteopontin in Cardiovascular Diseases
Abstract
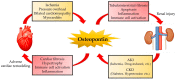
Unprecedented advances in secondary prevention have greatly improved the prognosis of cardiovascular diseases (CVDs); however, CVDs remain a leading cause of death globally. These findings suggest the need to reconsider cardiovascular risk and optimal medical therapy. Numerous studies have shown that inflammation, pro-thrombotic factors, and gene mutations are focused not only on cardiovascular residual risk but also as the next therapeutic target for CVDs. Furthermore, recent clinical trials, such as the Canakinumab Anti-inflammatory Thrombosis Outcomes Study trial, showed the possibility of anti-inflammatory therapy for patients with CVDs. Osteopontin (OPN) is a matricellular protein that mediates diverse biological functions and is involved in a number of pathological states in CVDs. OPN has a two-faced phenotype that is dependent on the pathological state. Acute increases in OPN have protective roles, including wound healing, neovascularization, and amelioration of vascular calcification. By contrast, chronic increases in OPN predict poor prognosis of a major adverse cardiovascular event independent of conventional cardiovascular risk factors. Thus, OPN can be a therapeutic target for CVDs but is not clinically available. In this review, we discuss the role of OPN in the development of CVDs and its potential as a therapeutic target.
Keywords: cardiovascular disease; inflammation; osteopontin.
Conflict of interest statement
All authors have read the journal authorship agreement and policy on disclosure of potential conflicts of interest and have nothing to disclose.
Figures

References
-
- Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

