The Phagosome-Lysosome Fusion Is the Target of a Purified Quillaja saponin Extract (PQSE) in Reducing Infection of Fish Macrophages by the Bacterial Pathogen Piscirickettsia salmonis
- PMID: 34356768
- PMCID: PMC8300623
- DOI: 10.3390/antibiotics10070847
The Phagosome-Lysosome Fusion Is the Target of a Purified Quillaja saponin Extract (PQSE) in Reducing Infection of Fish Macrophages by the Bacterial Pathogen Piscirickettsia salmonis
Abstract
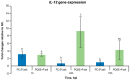
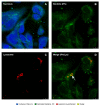
Piscirickettsia salmonis, the etiological agent of Piscirickettsiosis, is a Gram-negative and facultative intracellular pathogen that has affected the Chilean salmon industry since 1989. The bacterium is highly aggressive and can survive and replicate within fish macrophages using the Dot/Icm secretion system to evade the host's immune response and spread systemically. To date, no efficient control measures have been developed for this disease; therefore, the producers use large amounts of antibiotics to control this pathogen. In this frame, this work has focused on evaluating the use of saponins from Quillaja saponaria as a new alternative to control the Piscirickettsiosis. It has been previously reported that purified extract of Q. saponaria (PQSE) displays both antimicrobial activity against pathogenic bacteria and viruses and adjuvant properties. Our results show that PQSE does not present antimicrobial activity against P. salmonis, although it reduces P. salmonis infection in an in vitro model, promoting the phagosome-lysosome fusion. Additionally, we demonstrate that PQSE modulates the expression of IL-12 and IL-10 in infected cells, promoting the immune response against the pathogen and reducing the expression of pathogen virulence genes. These results together strongly argue for specific anti-invasion and anti-intracellular replication effects induced by the PQSE in macrophages.
Keywords: Piscirickettsia salmonis; Quillaja saponaria; bacterial; fish macrophage; phagosome–lysosome fusion; saponin.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study, decisions about protocols, developments of the trials, the collection of data, discussions, the analysis of results, the writing of the manuscript, or in the decision to publish the results.
Figures







Similar articles
-
In Vivo Efficacy of Purified Quillaja Saponin Extracts in Protecting against Piscirickettsia salmonis Infections in Atlantic Salmon (Salmo salar).Animals (Basel). 2023 Sep 7;13(18):2845. doi: 10.3390/ani13182845. Animals (Basel). 2023. PMID: 37760245 Free PMC article.
-
Non-Specific Antibodies Induce Lysosomal Activation in Atlantic Salmon Macrophages Infected by Piscirickettsia salmonis.Front Immunol. 2020 Nov 12;11:544718. doi: 10.3389/fimmu.2020.544718. eCollection 2020. Front Immunol. 2020. PMID: 33281810 Free PMC article.
-
Evidence of the presence of a functional Dot/Icm type IV-B secretion system in the fish bacterial pathogen Piscirickettsia salmonis.PLoS One. 2013;8(1):e54934. doi: 10.1371/journal.pone.0054934. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23383004 Free PMC article.
-
Piscirickettsiosis and Piscirickettsia salmonis in fish: a review.J Fish Dis. 2014 Mar;37(3):163-88. doi: 10.1111/jfd.12211. Epub 2013 Nov 26. J Fish Dis. 2014. PMID: 24279295 Review.
-
Why Does Piscirickettsia salmonis Break the Immunological Paradigm in Farmed Salmon? Biological Context to Understand the Relative Control of Piscirickettsiosis.Front Immunol. 2022 Mar 21;13:856896. doi: 10.3389/fimmu.2022.856896. eCollection 2022. Front Immunol. 2022. PMID: 35386699 Free PMC article. Review.
Cited by
-
Quercetin and Silybin Decrease Intracellular Replication of Piscirickettsia salmonis in SHK-1 Cell.Int J Mol Sci. 2025 Jan 29;26(3):1184. doi: 10.3390/ijms26031184. Int J Mol Sci. 2025. PMID: 39940952 Free PMC article.
-
In Vivo Efficacy of Purified Quillaja Saponin Extracts in Protecting against Piscirickettsia salmonis Infections in Atlantic Salmon (Salmo salar).Animals (Basel). 2023 Sep 7;13(18):2845. doi: 10.3390/ani13182845. Animals (Basel). 2023. PMID: 37760245 Free PMC article.
References
-
- Figueroa J., Cárcamo J., Yañez A., Olavarria V., Ruiz P., Manríquez R., Muñoz C., Romero A., Avendaño-Herrera R. Addressing viral and bacterial threats to salmon farming in Chile: Historical contexts and perspectives for management and control. Rev. Aquac. 2019;11:299–324. doi: 10.1111/raq.12333. - DOI
-
- Servicio Nacional de Pesca y Acuicultura (Sernapesca). Informe Sanitario de Salmonicultura en Centros Marinos. Primer Semestre; Valparaíso, Chile: 2018.
-
- Tandberg J.I., Lagos L.X., Langlete P., Berger E., Rishovd A.-L., Roos N., Varkey D., Paulsen I., Winther-Larsen H.C. Comparative analysis of membrane vesicles from three Piscirickettsia salmonis isolates reveals differences in vesicle characteristics. PloS ONE. 2016;11:e0165099. doi: 10.1371/journal.pone.0165099. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

