Glycobiology of the Epithelial to Mesenchymal Transition
- PMID: 34356834
- PMCID: PMC8301408
- DOI: 10.3390/biomedicines9070770
Glycobiology of the Epithelial to Mesenchymal Transition
Abstract
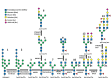
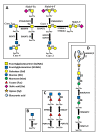
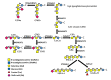
Glycosylation consists in the covalent, enzyme mediated, attachment of sugar chains to proteins and lipids. A large proportion of membrane and secreted proteins are indeed glycoproteins, while glycolipids are fundamental component of cell membranes. The biosynthesis of sugar chains is mediated by glycosyltransferases, whose level of expression represents a major factor of regulation of the glycosylation process. In cancer, glycosylation undergoes profound changes, which often contribute to invasion and metastasis. Epithelial to mesenchymal transition (EMT) is a key step in metastasis formation and is intimately associated with glycosylation changes. Numerous carbohydrate structures undergo up- or down-regulation during EMT and often regulate the process. In this review, we will discuss the relationship with EMT of the N-glycans, of the different types of O-glycans, including the classical mucin-type, O-GlcNAc, O-linked fucose, O-linked mannose and of glycolipids. Finally, we will discuss the role in EMT of galectins, a major class of mammalian galactoside-binding lectins. While the expression of specific carbohydrate structures can be used as a marker of EMT and of the propensity to migrate, the manipulation of the glycosylation machinery offers new perspectives for cancer treatment through inhibition of EMT.
Keywords: carbohydrate antigens; galectins; glycolipids; glycosylation; glycosyltransferases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

