T2-High Endotype and Response to Biological Treatments in Patients with Bronchiectasis
- PMID: 34356836
- PMCID: PMC8301446
- DOI: 10.3390/biomedicines9070772
T2-High Endotype and Response to Biological Treatments in Patients with Bronchiectasis
Abstract
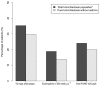
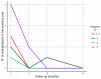
Although bronchiectasis pathophysiology has been historically understood around the presence of airway neutrophilic inflammation, recent experiences are consistent with the identification of a type 2 inflammation (T2) high endotype in bronchiectasis. In order to evaluate prevalence and clinical characteristics of bronchiectasis patients with a T2-high endotype and explore their response to biologicals, two studies were carried out. In a cross-sectional study, bronchiectasis adults without asthma underwent clinical, radiological, and microbiological assessment, along with blood eosinophils and oral fractional exhaled nitric oxide (FeNO) evaluation, during stable state. Prevalence and characteristics of patients with a T2- high endotype (defined by the presence of either eosinophils blood count ≥300 cells·µL-1 or oral FeNO ≥ 25 dpp) were reported. A case series of severe asthmatic patients with concomitant bronchiectasis treated with either mepolizumab or benralizumab was evaluated, and patients' clinical data pre- and post-treatment were analyzed up to 2 years of follow up. Among bronchiectasis patients without asthma enrolled in the cross-sectional study, a T2-high endotype was present in 31% of them. These patients exhibited a more severe disease, high dyspnea severity, low respiratory function, and high impact on quality of life. Among the five patients with severe eosinophilic asthma and concomitant bronchiectasis included in the series, treatment with either mepolizumab or benralizumab significantly reduced the exacerbation rate with an effect that persists for up to 2 years of follow up. If validated across different settings, our data suggest the need to design randomized controlled trials on biological treatments targeting the T2-high endotype in bronchiectasis patients.
Keywords: Bronchiectasis; T2-high; eosinophilia; type 2 inflammation.
Conflict of interest statement
M.O., F.A., A.G., A.D., M.G., M.C., F.B. (Francesco Bindo), C.D.F., M.S., E.S., and G.S. declare no conflict of interest. F.B. (Francesco Blasi) reports grants and personal fees from Astrazeneca, grants from Bayer, grants and personal fees from Chiesi, grants and personal fees from GlaxoSmithKline, personal fees from Grifols, personal fees from Guidotti, personal fees from Insmed, grants and personal fees from Menarini, personal fees from Novartis, grants and personal fees from Pfizer, personal fees from Zambon, personal fees from Vertex, outside the submitted work. S.A. reports personal fees from Bayer Healthcare, personal fees from Grifols, personal fees from AstraZeneca, personal fees from Zambon, grants and personal fees from Chiesi, grants and personal fees from INSMED, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees from ZetaCube Srl, grants from Fisher & Paykel, outside the submitted work.
Figures
References
-
- Polverino E., Goeminne P.C., McDonnell M.J., Aliberti S., Marshall S.E., Loebinger M.R., Murris-Espin M., Cantón R., Torres A., Dimakou K., et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017;50:1700629. doi: 10.1183/13993003.00629-2017. - DOI - PubMed
LinkOut - more resources
Full Text Sources

