MicroRNA-138 Increases Chemo-Sensitivity of Glioblastoma through Downregulation of Survivin
- PMID: 34356844
- PMCID: PMC8301402
- DOI: 10.3390/biomedicines9070780
MicroRNA-138 Increases Chemo-Sensitivity of Glioblastoma through Downregulation of Survivin
Abstract
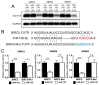
Glioblastoma (GBM) is one of the most deadly cancers and poorly responses to chemotherapies, such as temozolomide (TMZ). Dysregulation of intrinsic signaling pathways in cancer cells are often resulted by dysregulated tumor suppressive microRNAs (miRNAs). Previously, we found miR-138 as one of tumor suppressive miRNAs that were significantly down-regulated in GBM. In this study, we demonstrated that ectopic over-expression of miR-138 sensitizes GBM cells to the treatment of TMZ and increased apoptotic cell death. Mechanistically, miR-138 directly repressed the expression of Survivin, an anti-apoptotic protein, to enhance caspase-induced apoptosis upon TMZ treatment. Using an intracranial GBM xenograft mice model, we also showed that combination of miR-138 with TMZ increases survival rates of the mice compared to the control mice treated with TMZ alone. This study provides strong preclinical evidence of the therapeutic benefit from restoration of miR-138 to sensitize the GBM tumor to conventional chemotherapy.
Keywords: BIRC5; glioblastoma (GBM); microRNA-138 (miR-138); survivin; temozolomide (TMZ).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

