Effects of Glucosinolate-Derived Isothiocyanates on Fungi: A Comprehensive Review on Direct Effects, Mechanisms, Structure-Activity Relationship Data and Possible Agricultural Applications
- PMID: 34356918
- PMCID: PMC8305656
- DOI: 10.3390/jof7070539
Effects of Glucosinolate-Derived Isothiocyanates on Fungi: A Comprehensive Review on Direct Effects, Mechanisms, Structure-Activity Relationship Data and Possible Agricultural Applications
Abstract
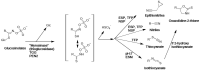
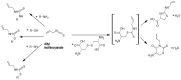
Plants heavily rely on chemical defense systems against a variety of stressors. The glucosinolates in the Brassicaceae and some allies are the core molecules of one of the most researched such pathways. These natural products are enzymatically converted into isothiocyanates (ITCs) and occasionally other defensive volatile organic constituents (VOCs) upon fungal challenge or tissue disruption to protect the host against the stressor. The current review provides a comprehensive insight on the effects of the isothiocyanates on fungi, including, but not limited to mycorrhizal fungi and pathogens of Brassicaceae. In the review, our current knowledge on the following topics are summarized: direct antifungal activity and the proposed mechanisms of antifungal action, QSAR (quantitative structure-activity relationships), synergistic activity of ITCs with other agents, effects of ITCs on soil microbial composition and allelopathic activity. A detailed insight into the possible applications is also provided: the literature of biofumigation studies, inhibition of post-harvest pathogenesis and protection of various products including grains and fruits is also reviewed herein.
Keywords: Cruciferae; QSAR; VOC; antifungal natural products; biofumigation; crop protection; fungi; glucosinolates; grain storage; isothiocyanates; synergistic activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Antifungal Activity of Glucosinolate-Derived Nitriles and Their Synergistic Activity with Glucosinolate-Derived Isothiocyanates Distinguishes Various Taxa of Brassicaceae Endophytes and Soil Fungi.Plants (Basel). 2023 Jul 24;12(14):2741. doi: 10.3390/plants12142741. Plants (Basel). 2023. PMID: 37514355 Free PMC article.
-
Interactions of fungi with non-isothiocyanate products of the plant glucosinolate pathway: A review on product formation, antifungal activity, mode of action and biotransformation.Phytochemistry. 2022 Aug;200:113245. doi: 10.1016/j.phytochem.2022.113245. Epub 2022 May 24. Phytochemistry. 2022. PMID: 35623473 Review.
-
Antifungal mechanism of isothiocyanates against Cochliobolus heterostrophus.Pest Manag Sci. 2022 Dec;78(12):5133-5141. doi: 10.1002/ps.7131. Epub 2022 Sep 7. Pest Manag Sci. 2022. PMID: 36053944
-
Degradation of Biofumigant Isothiocyanates and Allyl Glucosinolate in Soil and Their Effects on the Microbial Community Composition.PLoS One. 2015 Jul 17;10(7):e0132931. doi: 10.1371/journal.pone.0132931. eCollection 2015. PLoS One. 2015. PMID: 26186695 Free PMC article.
-
Brassicaceae Isothiocyanate-Mediated Alleviation of Soil-Borne Diseases.Plants (Basel). 2025 Apr 12;14(8):1200. doi: 10.3390/plants14081200. Plants (Basel). 2025. PMID: 40284088 Free PMC article. Review.
Cited by
-
Comparing Fungal Sensitivity to Isothiocyanate Products on Different Botrytis spp.Plants (Basel). 2024 Mar 7;13(6):756. doi: 10.3390/plants13060756. Plants (Basel). 2024. PMID: 38592765 Free PMC article.
-
Legacy effects of fumigation on soil bacterial and fungal communities and their response to metam sodium application.Environ Microbiome. 2022 Dec 3;17(1):59. doi: 10.1186/s40793-022-00454-w. Environ Microbiome. 2022. PMID: 36461097 Free PMC article.
-
Antifungal Activity of Glucosinolate-Derived Nitriles and Their Synergistic Activity with Glucosinolate-Derived Isothiocyanates Distinguishes Various Taxa of Brassicaceae Endophytes and Soil Fungi.Plants (Basel). 2023 Jul 24;12(14):2741. doi: 10.3390/plants12142741. Plants (Basel). 2023. PMID: 37514355 Free PMC article.
-
Comprehensive Review of Aflatoxin and Ochratoxin A Dynamics: Emergence, Toxicological Impact, and Advanced Control Strategies.Foods. 2024 Jun 18;13(12):1920. doi: 10.3390/foods13121920. Foods. 2024. PMID: 38928866 Free PMC article. Review.
-
The Role of the GSTF11 Gene in Resistance to Powdery Mildew Infection and Cold Stress.Plants (Basel). 2021 Dec 11;10(12):2729. doi: 10.3390/plants10122729. Plants (Basel). 2021. PMID: 34961200 Free PMC article.
References
-
- Pongrac P., Vogel-Mikuš K., Poschenrieder C., Barceló J., Tolrà R., Regvar M. Molecular Microbial Ecology of the Rhizosphere. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2013. Arbuscular Mycorrhiza in Glucosinolate-Containing Plants: The Story of the Metal Hyperaccumulator Noccaea (Thlaspi) praecox (Brassicaceae) pp. 1023–1032.
-
- Wittstock U., Kurzbach E., Herfurth A.M., Stauber E.J. Advances in Botanical Research. Volume 80. Academic Press; Cambridge, MA, USA: 2016. Glucosinolate Breakdown; pp. 125–169.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

