Recent Findings in Azaphilone Pigments
- PMID: 34356920
- PMCID: PMC8307326
- DOI: 10.3390/jof7070541
Recent Findings in Azaphilone Pigments
Abstract
Filamentous fungi are known to biosynthesize an extraordinary range of azaphilones pigments with structural diversity and advantages over vegetal-derived colored natural products such agile and simple cultivation in the lab, acceptance of low-cost substrates, speed yield improvement, and ease of downstream processing. Modern genetic engineering allows industrial production, providing pigments with higher thermostability, water-solubility, and promising bioactivities combined with ecological functions. This review, covering the literature from 2020 onwards, focuses on the state-of-the-art of azaphilone dyes, the global market scenario, new compounds isolated in the period with respective biological activities, and biosynthetic pathways. Furthermore, we discussed the innovations of azaphilone cultivation and extraction techniques, as well as in yield improvement and scale-up. Potential applications in the food, cosmetic, pharmaceutical, and textile industries were also explored.
Keywords: azaphilones; biotechnological tools; filamentous fungi; natural pigments; non-mycotoxigenic strains; production; regulatory issues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
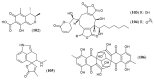

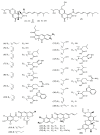
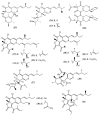

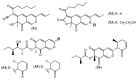

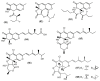


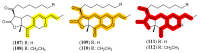

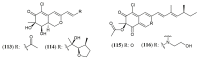
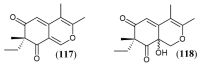
References
-
- Charlin V., Cifuentes A. A general framework to study the price-color relationship in paintings with an application to Mark Rothko rectangular series. Color Res. Appl. 2021;46:168–182. doi: 10.1002/col.22559. - DOI
-
- Labrecque L.I. Color research in marketing: Theoretical and technical considerations for conducting rigorous and impactful color research. Psychol. Mark. 2020;37:855–863. doi: 10.1002/mar.21359. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

