Sotorasib: First Approval
- PMID: 34357500
- PMCID: PMC8531079
- DOI: 10.1007/s40265-021-01574-2
Sotorasib: First Approval
Erratum in
-
Correction to: Sotorasib: First Approval.Drugs. 2021 Nov;81(16):1947. doi: 10.1007/s40265-021-01630-x. Drugs. 2021. PMID: 34687424 Free PMC article. No abstract available.
Abstract
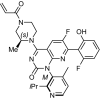
Sotorasib (LUMAKRAS™) is a RAS GTPase family inhibitor being developed by Amgen for the treatment of solid tumours with KRAS mutations, including non-small cell lung cancer (NSCLC) and colorectal cancer. In May 2021, sotorasib was granted accelerated approval by the US FDA for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy. This article summarizes the milestones in the development of sotorasib leading to this first approval for KRAS G12C-mutated NSCLC.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Hannah Blair is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
References
-
- Amgen. Amgen announces first clinical data evaluating novel investigational KRASG12C inhibitor AMG 510 at ASCO 2019 [media release]. 3 Jun 2019. https://www.amgen.com/newsroom/press-releases/2019/06/amgen-announces-fi....
-
- Amgen. Amgen's sotorasib granted breakthrough therapy designation for advanced or metastatic non-small cell lung cancer patients with KRAS G12C mutation [media release]. 8 Dec 2020. https://www.amgen.com/newsroom/press-releases/2020/12/amgens-sotorasib-g....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous