Repetitive saliva-based mass screening as a tool for controlling SARS-CoV-2 transmission in nursing homes
- PMID: 34357691
- PMCID: PMC8446975
- DOI: 10.1111/tbed.14280
Repetitive saliva-based mass screening as a tool for controlling SARS-CoV-2 transmission in nursing homes
Abstract
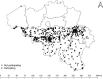
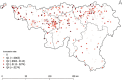
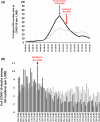
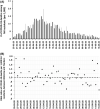
Nursing home (NH) residents and staff have been severely affected by the COVID-19 pandemic. The aim of this study was to examine the use of weekly saliva RT-qPCR testing for SARS-CoV-2 detection among NH workers as a strategy to control disease transmission within NHs in Belgium. From 16 November to 27 December 2020, a voluntary and anonymous weekly screening was implemented in a cohort of 50,000 workers across 572 NHs in the Walloon region of Belgium to detect asymptomatic cases of SARS-CoV-2 via saliva RT-qPCR testing and using the Diagenode saliva sample collection device. Positive workers were isolated to avoid subsequent infections in residents and other staff. RT-qPCR testing was based on pooled saliva sampling techniques from three workers, followed by individual testing of each positive or inconclusive pool. The majority of NHs (85%) and 55% of their workers participated. Pooling did not affect sensitivity as it only induced a very decrease in sensitivity estimated as 0.33%. Significant decreases in the prevalence (34.4-13.4%) and incidence of NHs with either single (13.8-2%) or multiple positive workers (3.7-0%) were observed over time. In addition, deaths among NH residents and NH worker absences decreased significantly over time. Weekly saliva RT-qPCR testing for SARS-CoV-2 demonstrated large-scale feasibility and efficacy in disrupting the chain of transmission. Implementation of this testing strategy in NHs could also be extended to other settings with the aim to control viral transmission for maintaining essential activities.
Keywords: Belgium; COVID-19; RT-qPCR; SARS-CoV-2; nursing home; saliva test; worker.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
The two last authors are the inventors of the device used in the saliva collection kit. This device was patented (EP20186086.3) and produced by Diagenode (Seraing, Belgium) under a commercial agreement with the University of Liège. This does not alter the adherence to all journal policies on sharing data and materials, as detailed online in the Author Guide.
Figures
References
-
- Czumbel, L. M. , Kiss, S. , Farkas, N. , Mandel, I. , Hegyi, A. , Nagy, Á. , Lohinai, Z. , Szakács, Z. , Hegyi, P. , Steward, M. C. , & Varga, G. (2020). Saliva as a candidate for COVID‐19 diagnostic testing: a meta‐analysis. Frontiers in Medicine, 7, 465. 10.3389/fmed.2020.00465 - DOI - PMC - PubMed
-
- Fox, J. , & Weisberg, S. (2018). An R Companion to Applied Regression. third edition. Sage, Thousand Oaks, CA:
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

