Melinacidin-Producing Acrostalagmus luteoalbus, a Major Constituent of Mixed Mycobiota Contaminating Insulation Material in an Outdoor Wall
- PMID: 34357993
- PMCID: PMC8308789
- DOI: 10.3390/pathogens10070843
Melinacidin-Producing Acrostalagmus luteoalbus, a Major Constituent of Mixed Mycobiota Contaminating Insulation Material in an Outdoor Wall
Abstract
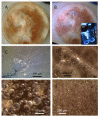
Occupants may complain about indoor air quality in closed spaces where the officially approved standard methods for indoor air quality risk assessment fail to reveal the cause of the problem. This study describes a rare genus not previously detected in Finnish buildings, Acrostalagmus, and its species A. luteoalbus as the major constituents of the mixed microbiota in the wet cork liner from an outdoor wall. Representatives of the genus were also present in the settled dust in offices where occupants suffered from symptoms related to the indoor air. One strain, POB8, was identified as A. luteoalbus by ITS sequencing. The strain produced the immunosuppressive and cytotoxic melinacidins II, III, and IV, as evidenced by mass spectrometry analysis. In addition, the classical toxigenic species indicating water damage, mycoparasitic Trichoderma, Aspergillus section Versicolores, Aspergillus section Circumdati, Aspergillus section Nigri, and Chaetomium spp., were detected in the wet outdoor wall and settled dust from the problematic rooms. The offices exhibited no visible signs of microbial growth, and the airborne load of microbial conidia was too low to explain the reported symptoms. In conclusion, we suggest the possible migration of microbial bioactive metabolites from the wet outdoor wall into indoor spaces as a plausible explanation for the reported complaints.
Keywords: Acrostalagmus luteoalbus; cork liner; guttation droplets; indoor dust; melinacidin; mixed mycobiota; outdoor wall.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Horve P., Lloyd S., Mhuireach G., Dietz L., Fretz M., MacCrone G., Van Den Wymelenberg K., Ishaq S.L. Building upon current knowledge and techniques of indoor microbiology to construct the next era of theory into microorganisms, health, and the built environment. J. Expo. Sci. Environ. Epidemiol. 2020;30:219–235. doi: 10.1038/s41370-019-0157-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

