Selection for Plastic, Pathogen-Inducible Recombination in a Red Queen Model with Diploid Antagonists
- PMID: 34358051
- PMCID: PMC8308896
- DOI: 10.3390/pathogens10070898
Selection for Plastic, Pathogen-Inducible Recombination in a Red Queen Model with Diploid Antagonists
Erratum in
-
Erratum: Rybnikov et al. Selection for Plastic, Pathogen-Inducible Recombination in a Red Queen Model with Diploid Antagonists. Pathogens 2021, 10, 898.Pathogens. 2021 Nov 11;10(11):1460. doi: 10.3390/pathogens10111460. Pathogens. 2021. PMID: 34832684 Free PMC article.
Abstract
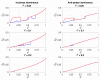
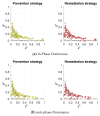
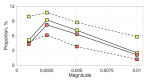
Antagonistic interactions and co-evolution between a host and its parasite are known to cause oscillations in the population genetic structure of both species (Red Queen dynamics). Potentially, such oscillations may select for increased sex and recombination in the host, although theoretical models suggest that this happens under rather restricted values of selection intensity, epistasis, and other parameters. Here, we explore a model in which the diploid parasite succeeds to infect the diploid host only if their phenotypes at the interaction-mediating loci match. Whenever regular oscillations emerge in this system, we test whether plastic, pathogen-inducible recombination in the host can be favored over the optimal constant recombination. Two forms of the host recombination dependence on the parasite pressure were considered: either proportionally to the risk of infection (prevention strategy) or upon the fact of infection (remediation strategy). We show that both forms of plastic recombination can be favored, although relatively infrequently (up to 11% of all regimes with regular oscillations, and up to 20% of regimes with obligate parasitism). This happens under either strong overall selection and high recombination rate in the host, or weak overall selection and low recombination rate in the host. In the latter case, the system's dynamics are considerably more complex. The prevention strategy is favored more often than the remediation one. It is noteworthy that plastic recombination can be favored even when any constant recombination is rejected, making plasticity an evolutionary mechanism for the rescue of host recombination.
Keywords: diploid selection; host-parasite co-evolution; matching-phenotype interaction; recombination plasticity; modifier model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Red Queen dynamics with non-standard fitness interactions.PLoS Comput Biol. 2009 Aug;5(8):e1000469. doi: 10.1371/journal.pcbi.1000469. Epub 2009 Aug 14. PLoS Comput Biol. 2009. PMID: 19680432 Free PMC article.
-
The evolution of sex and recombination in response to abiotic or coevolutionary fluctuations in epistasis.Genetics. 2007 Apr;175(4):1835-53. doi: 10.1534/genetics.106.066399. Epub 2007 Feb 4. Genetics. 2007. PMID: 17277371 Free PMC article.
-
Differences between selection on sex versus recombination in red queen models with diploid hosts.Evolution. 2009 Aug;63(8):2131-41. doi: 10.1111/j.1558-5646.2009.00695.x. Epub 2009 Mar 27. Evolution. 2009. PMID: 19453733
-
The coevolutionary dynamics of obligate ant social parasite systems--between prudence and antagonism.Biol Rev Camb Philos Soc. 2005 May;80(2):251-67. doi: 10.1017/s1464793104006669. Biol Rev Camb Philos Soc. 2005. PMID: 15921051 Review.
-
Deleterious mutations, variable epistatic interactions, and the evolution of recombination.Theor Popul Biol. 1997 Apr;51(2):134-47. doi: 10.1006/tpbi.1997.1301. Theor Popul Biol. 1997. PMID: 9169238 Review.
Cited by
-
Erratum: Rybnikov et al. Selection for Plastic, Pathogen-Inducible Recombination in a Red Queen Model with Diploid Antagonists. Pathogens 2021, 10, 898.Pathogens. 2021 Nov 11;10(11):1460. doi: 10.3390/pathogens10111460. Pathogens. 2021. PMID: 34832684 Free PMC article.
References
-
- Volterra V. Fluctuations in the Abundance of a Species considered Mathematically1. Nat. Cell Biol. 1926;118:558–560. doi: 10.1038/118558a0. - DOI
-
- De Bach P., Smith H.S. Are population oscillations inherent in the host-parasite relation? Ecology. 1941;22:363–369. doi: 10.2307/1930709. - DOI
-
- Stiven A.E. Experimental Studies on the Epidemiology of the Host Parasite System, Hydra and Hydramoeba hydroxena (Entz). II. The Components of a Simple Epidemic. Ecol. Monogr. 1964;34:119–142. doi: 10.2307/1948450. - DOI
-
- Van den Bosch R., Schlinger E.I., Lagace C.F., Hall J.C. Parasitization of Acyrthosiphon pisum by Aphidius smithi, a density-dependent process in nature (Homoptera: Aphidae) (Hymenoptera: Aphidiidae) Ecology. 1966;47:1049–1055. doi: 10.2307/1935655. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

