Insights into an Immunotherapeutic Approach to Combat Multidrug Resistance in Hepatocellular Carcinoma
- PMID: 34358082
- PMCID: PMC8308499
- DOI: 10.3390/ph14070656
Insights into an Immunotherapeutic Approach to Combat Multidrug Resistance in Hepatocellular Carcinoma
Abstract
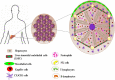
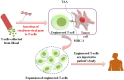
Hepatocellular carcinoma (HCC) has emerged as one of the most lethal cancers worldwide because of its high refractoriness and multi-drug resistance to existing chemotherapies, which leads to poor patient survival. Novel pharmacological strategies to tackle HCC are based on oral multi-kinase inhibitors like sorafenib; however, the clinical use of the drug is restricted due to the limited survival rate and significant side effects, suggesting the existence of a primary or/and acquired drug-resistance mechanism. Because of this hurdle, HCC patients are forced through incomplete therapy. Although multiple approaches have been employed in parallel to overcome multidrug resistance (MDR), the results are varying with insignificant outcomes. In the past decade, cancer immunotherapy has emerged as a breakthrough approach and has played a critical role in HCC treatment. The liver is the main immune organ of the lymphatic system. Researchers utilize immunotherapy because immune evasion is considered a major reason for rapid HCC progression. Moreover, the immune response can be augmented and sustained, thus preventing cancer relapse over the post-treatment period. In this review, we provide detailed insights into the immunotherapeutic approaches to combat MDR by focusing on HCC, together with challenges in clinical translation.
Keywords: hepatocellular carcinoma; immunotherapy; multidrug resistance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges.Oncologist. 2019 Feb;24(Suppl 1):S3-S10. doi: 10.1634/theoncologist.2019-IO-S1-s01. Oncologist. 2019. PMID: 30819826 Free PMC article. Review.
-
Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma.Front Immunol. 2021 Nov 26;12:783236. doi: 10.3389/fimmu.2021.783236. eCollection 2021. Front Immunol. 2021. PMID: 34899747 Free PMC article. Review.
-
New insights into the pharmacological, immunological, and CAR-T-cell approaches in the treatment of hepatocellular carcinoma.Drug Resist Updat. 2020 Jul;51:100702. doi: 10.1016/j.drup.2020.100702. Epub 2020 Apr 19. Drug Resist Updat. 2020. PMID: 32371296 Review.
-
Bibliometric study of immunotherapy for hepatocellular carcinoma.Front Immunol. 2023 Aug 4;14:1210802. doi: 10.3389/fimmu.2023.1210802. eCollection 2023. Front Immunol. 2023. PMID: 37600802 Free PMC article. Review.
-
Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells.PLoS One. 2017 Sep 21;12(9):e0185088. doi: 10.1371/journal.pone.0185088. eCollection 2017. PLoS One. 2017. PMID: 28934275 Free PMC article.
Cited by
-
Deciphering the role of transforming growth factor-beta 1 as a diagnostic-prognostic-therapeutic candidate against hepatocellular carcinoma.World J Gastroenterol. 2022 Sep 28;28(36):5250-5264. doi: 10.3748/wjg.v28.i36.5250. World J Gastroenterol. 2022. PMID: 36185626 Free PMC article. Review.
-
Blockade of Uttroside B-Induced Autophagic Pro-Survival Signals Augments Its Chemotherapeutic Efficacy Against Hepatocellular Carcinoma.Front Oncol. 2022 Feb 8;12:812598. doi: 10.3389/fonc.2022.812598. eCollection 2022. Front Oncol. 2022. PMID: 35211405 Free PMC article.
-
Translational Phytomedicines against Cancer: Promise and Hurdles.Adv Pharm Bull. 2023 Mar;13(2):210-215. doi: 10.34172/apb.2023.023. Epub 2022 Apr 28. Adv Pharm Bull. 2023. PMID: 37342376 Free PMC article. No abstract available.
-
Advancements in elucidating the mechanisms of Sorafenib resistance in hepatocellular carcinoma.Int J Surg. 2025 Apr 1;111(4):2990-3005. doi: 10.1097/JS9.0000000000002294. Int J Surg. 2025. PMID: 39992113 Free PMC article. Review.
-
Design and In Vitro Evaluation of Novel Cationic Lipids for siRNA Delivery in Breast Cancer Cell Lines.Evid Based Complement Alternat Med. 2022 Jun 6;2022:9231641. doi: 10.1155/2022/9231641. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Aug 2;2023:9892315. doi: 10.1155/2023/9892315. PMID: 35707479 Free PMC article. Retracted.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

