Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells
- PMID: 34358152
- PMCID: PMC8310084
- DOI: 10.3390/vaccines9070736
Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells
Abstract
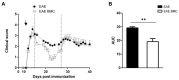
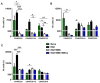
Multiple sclerosis (MS) is a neurological disorder characterized by an autoimmune response, demyelinating plaques and axonal damage. Intense immunosuppression (II) followed by autologous hematopoietic stem cell transplantation has been proposed as a treatment in severe forms of MS. We have used murine relapsing-remitting (RR) experimental autoimmune encephalomyelitis (RR-EAE) to evaluate the transplantation of syngeneic bone marrow cells (BMC) after II, in combination with mesenchymal stem cells (MSCs) as a new therapeutic adjunct capable of improving immune reconstitution. In EAE-affected mice treated with BMC alone, we observed a drastic reduction in the clinical course only during the early RR phase of the disease. There was no difference in the RR-EAE clinical course between mice treated with BMC alone and co-transplanted mice. To analyze the immune reconstitution, we quantified the circulating immune cells in naïve and RR-EAE-affected mice after II, with BMC alone or in combination with MSC. Although II resulted in reduced numbers of circulating immune cells, reconstitution did not differ in co-transplanted mice. During the early phase of the disease, IL-4 was significantly elevated in co-transplanted mice, as compared to those treated with BMC alone. These data suggest that BMC transplantation after II transiently ameliorates the clinical symptoms of RR-EAE, but that co-transplantation with MSC has no synergistic effect.
Keywords: bone-marrow cells; experimental autoimmune encephalomyelitis; intensive immunosuppression; mesenchymal stem cells; multiple sclerosis; stem cell therapy.
Conflict of interest statement
The authors have no conflict of interest to declare that are relevant to the content of this article.
Figures


Similar articles
-
Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors.Immunol Lett. 2013 Jul-Aug;154(1-2):70-6. doi: 10.1016/j.imlet.2013.06.002. Epub 2013 Aug 28. Immunol Lett. 2013. PMID: 23994102
-
Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice.Glia. 2010 Mar;58(4):434-45. doi: 10.1002/glia.20935. Glia. 2010. PMID: 19780195
-
CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells.Exp Neurol. 2017 Sep;295:222-232. doi: 10.1016/j.expneurol.2017.06.013. Epub 2017 Jun 9. Exp Neurol. 2017. PMID: 28602834 Free PMC article.
-
Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model.IUBMB Life. 2016 Feb;68(2):106-15. doi: 10.1002/iub.1469. Epub 2016 Jan 12. IUBMB Life. 2016. PMID: 26757144 Review.
-
A Case of Multiple Sclerosis-Like Relapsing Remitting Encephalomyelitis Following Allogeneic Hematopoietic Stem Cell Transplantation and a Review of the Published Literature.Front Immunol. 2020 May 5;11:668. doi: 10.3389/fimmu.2020.00668. eCollection 2020. Front Immunol. 2020. PMID: 32431694 Free PMC article. Review.
References
-
- Muraro P.A., Pasquini M., Atkins H.L., Bowen J.D., Farge D., Fassas A., Freedman M.S., Georges G.E., Gualandi F., Hamerschlak N., et al. Long-term Outcomes After Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis. JAMA Neurol. 2017;74:459–469. doi: 10.1001/jamaneurol.2016.5867. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

