Proteins as Targets in Anti-Schistosomal Drug Discovery and Vaccine Development
- PMID: 34358178
- PMCID: PMC8310332
- DOI: 10.3390/vaccines9070762
Proteins as Targets in Anti-Schistosomal Drug Discovery and Vaccine Development
Abstract
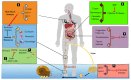
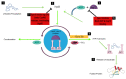
Proteins hardly function in isolation; they form complexes with other proteins or molecules to mediate cell signaling and control cellular processes in various organisms. Protein interactions control mechanisms that lead to normal and/or disease states. The use of competitive small molecule inhibitors to disrupt disease-relevant protein-protein interactions (PPIs) holds great promise for the development of new drugs. Schistosome invasion of the human host involves a variety of cross-species protein interactions. The pathogen expresses specific proteins that not only facilitate the breach of physical and biochemical barriers present in skin, but also evade the immune system and digestion of human hemoglobin, allowing for survival in the host for years. However, only a small number of specific protein interactions between the host and parasite have been functionally characterized; thus, in-depth understanding of the molecular mechanisms of these interactions is a key component in the development of new treatment methods. Efforts are now focused on developing a schistosomiasis vaccine, as a proposed better strategy used either alone or in combination with Praziquantel to control and eliminate this disease. This review will highlight protein interactions in schistosomes that can be targeted by specific PPI inhibitors for the design of an alternative treatment to Praziquantel.
Keywords: Sm-P80/Gla-Se; Sm-Tsp-2/Alhydrogel; Sm14/Gla-Se; praziquantel; protein–protein interactions; schistosomiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection.Parasite Epidemiol Control. 2020 Aug 20;11:e00176. doi: 10.1016/j.parepi.2020.e00176. eCollection 2020 Nov. Parasite Epidemiol Control. 2020. PMID: 32923703 Free PMC article. Review.
-
Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons.Vaccine. 2014 Mar 5;32(11):1296-303. doi: 10.1016/j.vaccine.2013.12.057. Epub 2014 Jan 4. Vaccine. 2014. PMID: 24397898 Free PMC article.
-
Molecular Context of ADP-ribosylation in Schistosomes for Drug Discovery and Vaccine Development.Curr Drug Discov Technol. 2021;18(4):473-484. doi: 10.2174/1570163817666200806170654. Curr Drug Discov Technol. 2021. PMID: 32767945 Review.
-
Signalling pathways in schistosomes: novel targets for control interventions against schistosomiasis.Emerg Top Life Sci. 2017 Dec 22;1(6):633-639. doi: 10.1042/ETLS20170093. Emerg Top Life Sci. 2017. PMID: 33525854
-
A Review of Nanotechnology for Targeted Anti-schistosomal Therapy.Front Bioeng Biotechnol. 2020 Jan 31;8:32. doi: 10.3389/fbioe.2020.00032. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32083071 Free PMC article. Review.
Cited by
-
A genome-scale drug discovery pipeline uncovers new therapeutic targets and a unique p97 allosteric binding site in Schistosoma mansoni.bioRxiv [Preprint]. 2025 Mar 15:2025.03.14.643303. doi: 10.1101/2025.03.14.643303. bioRxiv. 2025. PMID: 40161785 Free PMC article. Preprint.
-
Metallic Nanoparticles and Core-Shell Nanosystems in the Treatment, Diagnosis, and Prevention of Parasitic Diseases.Pathogens. 2023 Jun 17;12(6):838. doi: 10.3390/pathogens12060838. Pathogens. 2023. PMID: 37375528 Free PMC article. Review.
-
Identification and characterization of a target antigen recognized by the monoclonal antibody against Opisthorchis viverrini.PLoS One. 2025 May 29;20(5):e0324137. doi: 10.1371/journal.pone.0324137. eCollection 2025. PLoS One. 2025. PMID: 40440420 Free PMC article.
-
Therapeutic and vaccinomic potential of moonlighting proteins for the discovery and design of drugs and vaccines against schistosomiasis.Am J Transl Res. 2024 Sep 15;16(9):4279-4300. doi: 10.62347/BXRT7210. eCollection 2024. Am J Transl Res. 2024. PMID: 39398578 Free PMC article. Review.
-
miR-182-5p attenuates Schistosoma japonicum-induced hepatic fibrosis by targeting tristetraprolin.Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(10):1421-1430. doi: 10.3724/abbs.2022130. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36148947 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

