A Pseudomonas aeruginosa-Derived Particulate Vaccine Protects against P. aeruginosa Infection
- PMID: 34358220
- PMCID: PMC8309987
- DOI: 10.3390/vaccines9070803
A Pseudomonas aeruginosa-Derived Particulate Vaccine Protects against P. aeruginosa Infection
Abstract
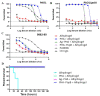
Despite numerous efforts to develop an effective vaccine against Pseudomonas aeruginosa, no vaccine has yet been approved for human use. This study investigates the utility of the P. aeruginosa inherently produced polyhydroxyalkanaote (PHA) inclusions and associated host-cell proteins (HCP) as a particulate vaccine platform. We further engineered PHA inclusions to display epitopes derived from the outer membrane proteins OprF/OprI/AlgE (Ag) or the type III secretion system translocator PopB. PHA and engineered PHA beads induced antigen-specific humoral, cell-mediated immune responses, anti-HCP and anti-polysaccharide Psl responses in mice. Antibodies mediated opsonophagocytic killing and serotype-independent protective immunity as shown by 100% survival upon challenge with P. aeruginosa in an acute pneumonia murine model. Vaccines were stable at 4 °C for at least one year. Overall, our data suggest that vaccination with subcellular empty PHA beads was sufficient to elicit multiple immune effectors that can prevent P. aeruginosa infection.
Keywords: Pseudomonas aeruginosa; polyhydroxyalkanoate; protective immunity; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
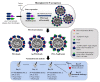
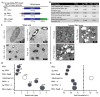
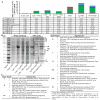
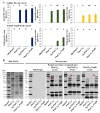
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

