Investigation of RNA metabolism through large-scale genetic interaction profiling in yeast
- PMID: 34358317
- PMCID: PMC8421204
- DOI: 10.1093/nar/gkab680
Investigation of RNA metabolism through large-scale genetic interaction profiling in yeast
Abstract
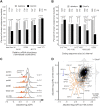
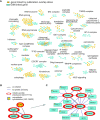
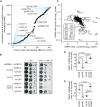
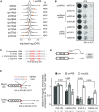
Gene deletion and gene expression alteration can lead to growth defects that are amplified or reduced when a second mutation is present in the same cells. We performed 154 genetic interaction mapping (GIM) screens with query mutants related with RNA metabolism and estimated the growth rates of about 700 000 double mutant Saccharomyces cerevisiae strains. The tested targets included the gene deletion collection and 900 strains in which essential genes were affected by mRNA destabilization (DAmP). To analyze the results, we developed RECAP, a strategy that validates genetic interaction profiles by comparison with gene co-citation frequency, and identified links between 1471 genes and 117 biological processes. In addition to these large-scale results, we validated both enhancement and suppression of slow growth measured for specific RNA-related pathways. Thus, negative genetic interactions identified a role for the OCA inositol polyphosphate hydrolase complex in mRNA translation initiation. By analysis of suppressors, we found that Puf4, a Pumilio family RNA binding protein, inhibits ribosomal protein Rpl9 function, by acting on a conserved UGUAcauUA motif located downstream the stop codon of the RPL9B mRNA. Altogether, the results and their analysis should represent a useful resource for discovery of gene function in yeast.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Dujon B.Yeast evolutionary genomics. Nat. Rev. Genet. 2010; 11:512–524. - PubMed
-
- Marsit S., Leducq J.-B., Durand E., Marchant A., Filteau M., Landry C.R.. Evolutionary biology through the lens of budding yeast comparative genomics. Nat. Rev. Genet. 2017; 18:581–598. - PubMed
-
- Gu Z., Steinmetz L.M., Gu X., Scharfe C., Davis R.W., Li W.-H.. Role of duplicate genes in genetic robustness against null mutations. Nature. 2003; 421:63–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

