Once-nightly sodium oxybate (FT218) demonstrated improvement of symptoms in a phase 3 randomized clinical trial in patients with narcolepsy
- PMID: 34358324
- PMCID: PMC9189976
- DOI: 10.1093/sleep/zsab200
Once-nightly sodium oxybate (FT218) demonstrated improvement of symptoms in a phase 3 randomized clinical trial in patients with narcolepsy
Abstract
Study objectives: To assess the efficacy and safety of FT218, a novel once-nightly formulation of sodium oxybate (ON-SXB), in patients with narcolepsy in the phase 3 REST-ON trial.
Methods: Narcolepsy patients aged ≥16 years were randomized 1:1 to uptitration of ON-SXB (4.5, 6, 7.5, and 9 g) or placebo. Three coprimary endpoints were change from baseline in mean sleep latency on the Maintenance of Wakefulness Test, Clinical Global Impression-Improvement rating, and weekly cataplexy attacks at 9, 7.5, and 6 g. Secondary endpoints included change from baseline on the Epworth Sleepiness Scale. Safety included adverse drug reactions and clinical laboratory assessments.
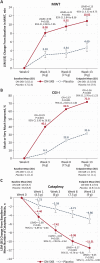
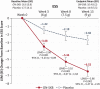
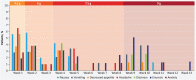
Results: In total, 222 patients were randomized; 212 received ≥1 dose of ON-SXB (n = 107) or placebo (n = 105). For the three coprimary endpoints and Epworth Sleepiness Scale, all three doses of ON-SXB demonstrated clinically meaningful, statistically significant improvement versus placebo (all p < 0.001). For ON-SXB 9 g versus placebo, increase in mean sleep latency was 10.8 versus 4.7 min (Least squares mean difference, LSMD [95% CI], 6.13 [3.52 to 8.75]), 72.0% versus 31.6% were rated much/very much improved on Clinical Global Impression-Improvement (OR [95% CI], 5.56 [2.76 to 11.23]), change in mean weekly number of cataplexy attacks was -11.5 versus -4.9 (LSMD [95% CI], -6.65 [-9.32 to -3.98]), and change in Epworth Sleepiness Scale was -6.5 and -2.7 (LSMD [95% CI], -6.52 [-5.47 to -2.26]). Common adverse reactions included nausea, vomiting, headache, dizziness, and enuresis.
Conclusions: ON-SXB significantly improved narcolepsy symptoms; its safety profile was consistent with SXB. ON-SXB conferred efficacy with a clearly beneficial single nighttime dose.
Clinical trial registration: ClinicalTrials.gov: NCT02720744, https://clinicaltrials.gov/ct2/show/NCT02720744.
Keywords: NT1/NT2; modified release; narcolepsy; once-nightly; safety; sodium oxybate.
© Sleep Research Society 2021. Published by Oxford University Press on behalf of the Sleep Research Society.
Figures

Comment in
-
Response to: Once-nightly sodium oxybate (FT218) in the treatment of narcolepsy: a letter to the editor commenting on the recent publication by C. Kushida et al.Sleep. 2022 Jun 13;45(6):zsac042. doi: 10.1093/sleep/zsac042. Sleep. 2022. PMID: 35695179 Free PMC article. No abstract available.
-
Once-nightly sodium oxybate (FT218) in the treatment of narcolepsy: a letter to the editor commenting on the recent publication by C. Kushida et al.Sleep. 2022 Jun 13;45(6):zsab292. doi: 10.1093/sleep/zsab292. Sleep. 2022. PMID: 35695180 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

