Chemical Modulation of Gasdermin-Mediated Pyroptosis and Therapeutic Potential
- PMID: 34358546
- PMCID: PMC8810912
- DOI: 10.1016/j.jmb.2021.167183
Chemical Modulation of Gasdermin-Mediated Pyroptosis and Therapeutic Potential
Abstract
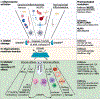
Pyroptosis, a lytic form of programmed cell death, both stimulates effective immune responses and causes tissue damage. Gasdermin (GSDM) proteins are a family of pore-forming executors of pyroptosis. While the most-studied member, GSDMD, exerts critical functions in inflammasome biology, emerging evidence demonstrates potential broad relevance for GSDM-mediated pyroptosis across diverse pathologies. In this review, we describe GSDM biology, outline conditions where inflammasomes and GSDM-mediated pyroptosis represent rational therapeutic targets, and delineate strategies to manipulate these central immunologic processes for the treatment of human disease.
Keywords: anti-tumor immunity; immunogenic cell death; inflammasome; inflammasomopathy; pore-forming protein.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

