Sudden Cardiac Death Caused by a Fatal Association of Hypertrophic Cardiomyopathy (MYH7, p.Arg719Trp), Heterozygous Familial Hypercholesterolemia (LDLR, p.Gly343Lys) and SARS-CoV-2 B.1.1.7 Infection
- PMID: 34359312
- PMCID: PMC8307649
- DOI: 10.3390/diagnostics11071229
Sudden Cardiac Death Caused by a Fatal Association of Hypertrophic Cardiomyopathy (MYH7, p.Arg719Trp), Heterozygous Familial Hypercholesterolemia (LDLR, p.Gly343Lys) and SARS-CoV-2 B.1.1.7 Infection
Abstract
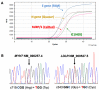
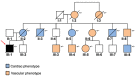
Hypertrophic cardiomyopathy (HCM) and heterozygous familial hypercholesterolemia (HeFH), two of the most common genetic cardiovascular disorders, can lead to sudden cardiac death. These conditions could be complicated by concomitant severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as in the case herein described. A young amateur soccer player died in late October 2020 after a fatal arrhythmia and the autopsy revealed the presence of HCM with diffuse non-obstructive coronary disease. The molecular autopsy revealed a compound condition with a first mutation in the MYH7 gene (p.Arg719Trp) and a second mutation in the LDLR gene (p.Gly343Cys): both have already been described as associated with HCM and HeFH, respectively. In addition, molecular analyses showed the presence of SARS-CoV-2 lineage B.1.1.7 (UK variant with high titer in the myocardium. Co-segregation analysis within the family (n = 19) showed that heterozygous LDLR mutation was maternally inherited, while the heterozygous MYH7 genetic lesion was de novo. All family member carriers of the LDLR mutation (n = 13) had systematic higher LDL plasma concentrations and positive records of cardiac and vascular ischemic events at young age. Considering that HCM mutations are in themselves involved in the predisposition to malignant arrhythmogenicity and HeFH could cause higher risk of cardiac complications in SARS-CoV-2 infection, this case could represent an example of a potential SARS-CoV-2 infection role in triggering or unmasking inherited cardiovascular disease, whose combination might represent the cause of fatal arrhythmia at such a young age. Additionally, it can provide clues in dating the presence of the SARS-CoV-2 lineage B.1.1.7 in Northern Italy in the early phases of the second pandemic wave.
Keywords: LDLR; MYH7; SARS-CoV-2; familial hypercholesterolemia; fatal arrhythmia; hypertrophic cardiomyopathy; lineage B.1.1.7.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zamorano J.L., Anastasakis A., Borger M.A., Borggrefe M., Cecchi F., Charron P., Hagege A.A., Lafont A., Limongelli G., Mahrholdt H., et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) Eur. Heart J. 2014;35:2733–2779. - PubMed
-
- Basso C., Leone O., Rizzo S., De Gaspari M., Van Der Wal A.C., Aubry M.C., Bois M.C., Lin P.T., Maleszewski J.J., Stone J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

