Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity
- PMID: 34359398
- PMCID: PMC8303315
- DOI: 10.3390/foods10071528
Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity
Abstract
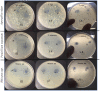
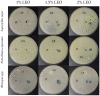
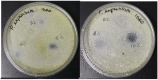
The use of natural compounds as food preservatives is becoming increasingly popular as it is perceived positively by consumers. Among these substances, essential oils have attracted great interest owing to their antioxidant and antimicrobial properties. However, several challenges impair the use of essential oils in food products, such as their degradation or loss during food processing and storage, the strong aroma, even at low concentrations, which may negatively affect the sensory characteristics of food. In this context, the development of nanoformulations able to stabilize essential oils may represent a smart solution to this issue. The aim of the study was to evaluate the efficiency of alginate-based nanoformulations enriched with lemongrass (Cymbopogon nardus) essential oil (LEO) and Tween 80 against several fungi namely Penicillium expansus, Aspergillus niger and Rhizopus spp. Firstly, the flow behavior of systems at different concentrations of alginate (1%, 2% and 3% w/w) were studied. Then, emulsion-based nanoformulations at different concentrations of lemongrass essential oil in the range of 0-2% w/w were stabilized by a fixed amount of Tween 80, characterized and tested for their antifungal activity. Our results showed that the best nanoformulation able to inhibit Rhizopus spp., Penicillium expansum and Aspergillus niger, for at least 10 days, was constituted by 1% alginate/1.5% LEO/1% Tween 80. Hence, the incorporation of essential oil into nanoformulation systems may represent a valid alternative to overcome the disadvantages that limit the commercial application of essential oils.
Keywords: Aspergillus niger; Penicillium expansum; Rhyzopus spp.; alginate; antimicrobial; emulsion; essential oil; moulds; nanoformulations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- De Lavor É.M., Fernandes A.W.C., de Andrade Teles R.B., Leal A.E.B.P., de Oliveira Júnior R.G., Gama e Silva M., De Oliveira A.P., Silva J.C., de Moura Fontes Araújo M.T., Coutinho H.D.M. Essential oils and their major compounds in the treatment of chronic inflammation: A review of antioxidant potential in preclinical studies and molecular mechanisms. Oxidative Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/6468593. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

