Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells
- PMID: 34359504
- PMCID: PMC8305880
- DOI: 10.3390/foods10071634
Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells
Abstract
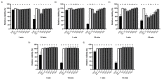
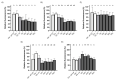
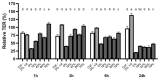
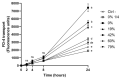
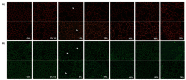
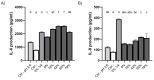
Cranberry (Vaccinium macrocarpon) may be a potent natural adjuvant for the prevention of oral diseases due to its anti-adherence, anti-cariogenic, and anti-inflammatory properties. However, the high titrable acidity of cranberry juice (CJ) has been reported to cause gastrointestinal discomfort, leading consumers to restrict their intake of this beverage. Electrodialysis with a bipolar membrane (EDBM) can reduce the organic acid content of CJ while retaining the flavonoids associated with potential health benefits. This study aimed to assess how the deacidification of CJ by EDBM impacts the antibacterial properties of the beverage against cariogenic (Streptococcus mutans, Streptococcus sobrinus) and commensal (Streptococcus gordonii, Streptococcus oralis, Streptococcus salivarius) streptococci, and how it affects oral epithelial barrier function and inflammatory response in an in vitro model. The removal of organic acids from CJ (deacidification rate ≥42%) reduced the bactericidal activity of the beverage against planktonic S. mutans and S. gordonii after a 15-min exposure, whereas only the viability of S. gordonii was significantly impacted by CJ deacidification rate when the bacteria were embedded in a biofilm. Moreover, conditioning saliva-coated hydroxyapatite with undiluted CJ samples significantly lowered the adherence of S. mutans, S. sobrinus, and S. oralis. With respect to epithelial barrier function, exposure to CJ deacidified at a rate of ≥19% maintained the integrity of a keratinocyte monolayer over the course of 24 h compared to raw CJ, as assessed by the determination of transepithelial electrical resistance (TER) and fluorescein isothiocyanate-conjugated dextran paracellular transport. These results can be in part attributed to the inability of the deacidified CJ to disrupt two tight junction proteins, zonula occludens-1 and occludin, following exposure, unlike raw CJ. Deacidification of CJ impacted the secretion of IL-6, but not of IL-8, by oral epithelial cells. In conclusion, deacidification of CJ appears to provide benefits with respect to the maintenance of oral health.
Keywords: antibacterial; cranberry juice; dental caries; electrodialysis; epithelial barrier; inflammation; oral streptococci; organic acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effect of cranberry juice deacidification on its antibacterial activity against periodontal pathogens and its anti-inflammatory properties in an oral epithelial cell model.Food Funct. 2021 Nov 1;12(21):10470-10483. doi: 10.1039/d1fo01552d. Food Funct. 2021. PMID: 34554173
-
Influence of cranberry juice on glucan-mediated processes involved in Streptococcus mutans biofilm development.Caries Res. 2006;40(1):20-7. doi: 10.1159/000088901. Caries Res. 2006. PMID: 16352876
-
The Concentration of Organic Acids in Cranberry Juice Modulates the Gut Microbiota in Mice.Int J Mol Sci. 2021 Oct 26;22(21):11537. doi: 10.3390/ijms222111537. Int J Mol Sci. 2021. PMID: 34768966 Free PMC article.
-
Potential oral health benefits of cranberry.Crit Rev Food Sci Nutr. 2008 Aug;48(7):672-80. doi: 10.1080/10408390701636211. Crit Rev Food Sci Nutr. 2008. PMID: 18663617 Review.
-
Cranberry Polyphenols: Natural Weapons against Dental Caries.Dent J (Basel). 2019 Mar 1;7(1):20. doi: 10.3390/dj7010020. Dent J (Basel). 2019. PMID: 30823634 Free PMC article. Review.
Cited by
-
Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties.Plants (Basel). 2025 May 5;14(9):1390. doi: 10.3390/plants14091390. Plants (Basel). 2025. PMID: 40364419 Free PMC article. Review.
References
Grants and funding
- 2019-2020/Laboratoire de Contrôle microbiologique of Université Laval
- IRCPJ 492889-15/NSERC Industrial Research Chair on ElectroMembrane processes aiming improvement of the ecoefficiency of biofood production lines
- 2017-072-C34/Consortium de Recherche et Innovations en Bioprocédés Industriels au Québec
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

