Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors
- PMID: 34359596
- PMCID: PMC8345067
- DOI: 10.3390/cancers13153695
Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors
Abstract
Invasive lobular breast cancer (ILC) is the most common special histological type of breast cancer (BC). This review recapitulates developments in the histomorphologic assessment of ILC from its beginnings with the seminal work of Foote and Stewart, which was published in 1941, until today. We discuss different concepts of ILC and their implications. These concepts include (i) BC arising from mammary lobules, (ii) BC growing in dissociated cells and single files, and (iii) BC defined as a morpho-molecular spectrum of tumors with distinct histological and molecular characteristics related to impaired cell adhesion. This review also provides a comprehensive overview of ILC variants, their histomorphology, and differential diagnosis. Furthermore, this review highlights recent advances which have contributed to a better understanding of the histomorphology of ILC, such as the role of the basal lamina component laminin, the molecular specificities of triple-negative ILC, and E-cadherin to P-cadherin expression switching as the molecular determinant of tubular elements in CDH1-deficient ILC. Last but not least, we provide a detailed account of the tumor microenvironment in ILC, including tumor infiltrating lymphocyte (TIL) levels, which are comparatively low in ILC compared to other BCs, but correlate with clinical outcome. The distinct histomorphology of ILC clearly reflects a special tumor biology. In the clinic, special treatment strategies have been established for triple-negative, HER2-positive, and ER-positive BC. Treatment specialization for patients diagnosed with ILC is just in its beginnings. Accordingly, ILC deserves greater attention as a special tumor entity in BC diagnostics, patient care, and cancer research.
Keywords: HER2; LCIS; LIN; beta-catenin; p120-catenin; pleomorphic; solid; tubulolobular.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures

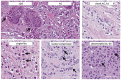
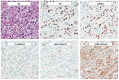
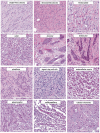

References
-
- Hoadley K.A., Yau C., Hinoue T., Wolf D.M., Lazar A.J., Drill E., Shen R., Taylor A.M., Cherniack A.D., Thorsson V., et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell. 2018;173:291–304.e296. doi: 10.1016/j.cell.2018.03.022. - DOI - PMC - PubMed
-
- Cheatle G.L., Cutler M. Tumors of the Breast. Their Pathology, Symptoms, Diagnosis and Treatment. Lippincott Company; Philadelphia, PA, USA: Montreal, QC, Canada: 1930.
-
- Cornil V. Les Tumeurs du Sein. Librairie Germer Bailliere and Co.; Paris, France: 1908.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

